Structure and Bonding Based on McMurry's Organic Chemistry,6th edition, Chapter 1
Structure and Bonding Based on McMurry’s Organic Chemistry, 6th edition, Chapter 1

Organic Chemistry A study of carbon compounds H He Be B Ne Na Mg Al Si Ar Sc Ti Cr Mn Fe Co Ni Cu Ga Ge Se Br Kr Rb Sr Zr Nb Sn Xe Cs Ba La Hf Ta Re Os Ir Au Hg T Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt 2004 Thon Although C is the principal element in organic compounds,most also contain H,and many contain N,O,P,S.CI or other elements
Organic Chemistry A study of carbon compounds Although C is the principal element in organic compounds, most also contain H, and many contain N, O, P, S. Cl or other elements
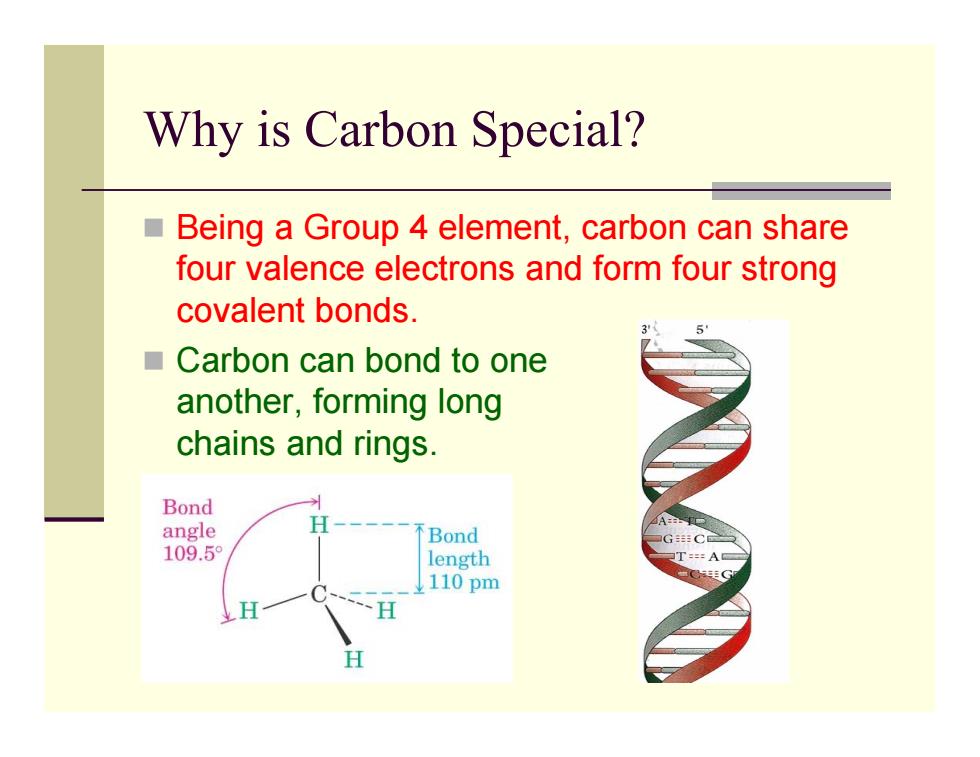
Why is Carbon Special? ■ Being a Group 4 element,carbon can share four valence electrons and form four strong covalent bonds. ■Carbon can bond to one another,forming long chains and rings. Bond angle Bond GC 109.5 length 110pm H
Why is Carbon Special? Being a Group 4 element, carbon can share four valence electrons and form four strong covalent bonds. Carbon can bond to one another, forming long chains and rings
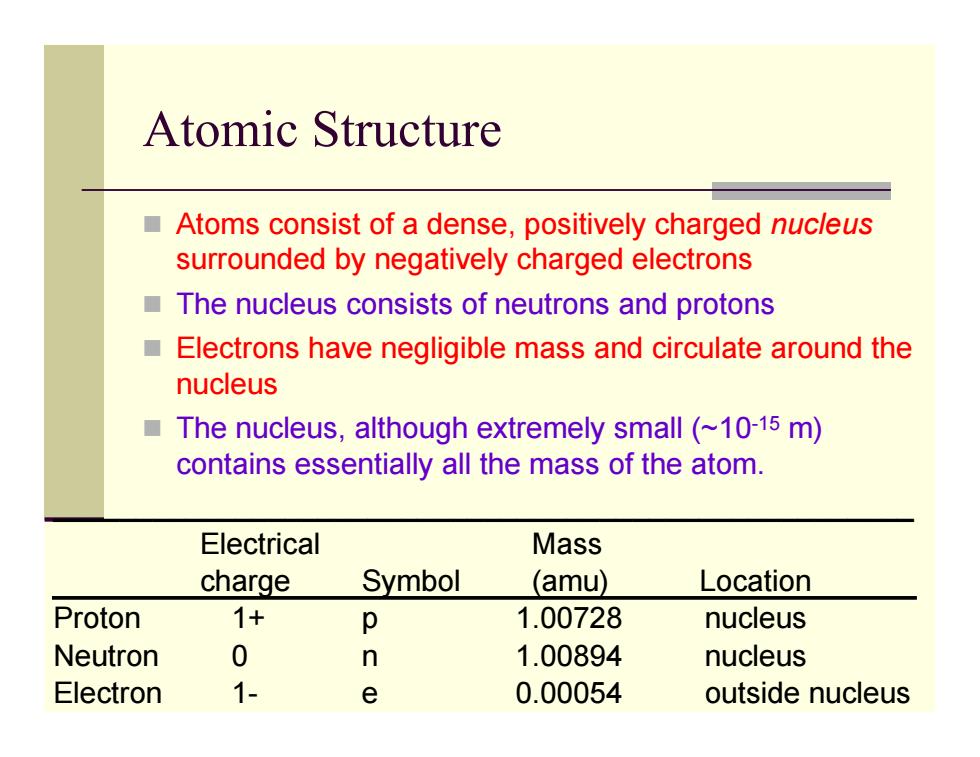
Atomic Structure Atoms consist of a dense,positively charged nucleus surrounded by negatively charged electrons ■ The nucleus consists of neutrons and protons ■ Electrons have negligible mass and circulate around the nucleus ■ The nucleus,although extremely small(~10-15 m) contains essentially all the mass of the atom. Electrical Mass charge Symbol (amu) Location Proton 1+ p 1.00728 nucleus Neutron 0 n 1.00894 nucleus Electron 1- e 0.00054 outside nucleus
Atomic Structure Atoms consist of a dense, positively charged nucleus surrounded by negatively charged electrons The nucleus consists of neutrons and protons Electrons have negligible mass and circulate around the nucleus The nucleus, although extremely small (~10-15 m) contains essentially all the mass of the atom. ____________________________________________________ Electrical Mass charge Symbol (amu) Location Proton 1+ p 1.00728 nucleus Neutron 0 n 1.00894 nucleus Electron 1- e 0.00054 outside nucleus

Atomic Number and Atomic Mass The atomic number(Z)is the number of protons in the atom's nucleus The mass number (A)is the number of protons plus neutrons All the atoms of a given element have the same atomic number ■ Isotopes are atoms of the same element that have different numbers of neutrons and therefore different mass numbers The atomic mass of an element is the weighted average mass in atomic mass units (amu)of an element's naturally occurring isotopes
Atomic Number and Atomic Mass The atomic number ( Z) is the number of protons in the atom's nucleus The mass number ( A) is the number of protons plus neutrons All the atoms of a given element have the same atomic number Isotopes are atoms of the same element that have different numbers of neutrons and therefore different mass numbers The atomic mass of an element is the weighted average mass in atomic mass units (amu) of an element’s naturally occurring isotopes