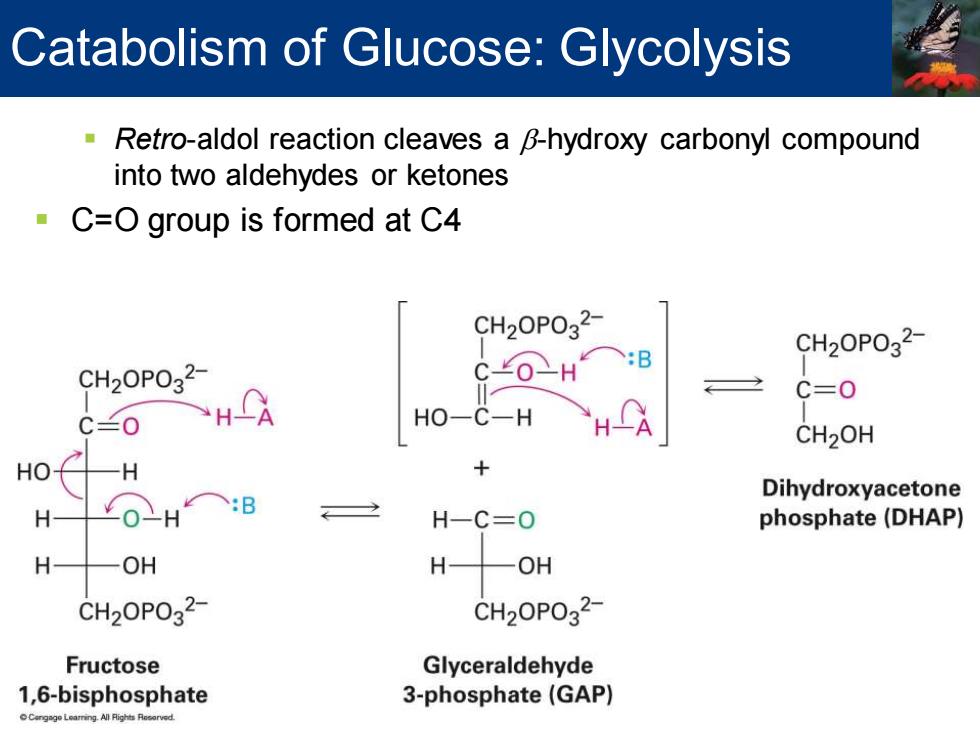
Catabolism of Glucose:Glycolysis Retro-aldol reaction cleaves a B-hydroxy carbonyl compound into two aldehydes or ketones C=O group is formed at C4 CH20P032- B CH20P032 CH20P032 C0-H C=0 C兰0 HO-C-H CH2OH HO -H Dihydroxyacetone H H-C=0 phosphate(DHAP) H OH H -OH CH20P032 CH20P032 Fructose Glyceraldehyde 1,6-bisphosphate 3-phosphate(GAP) Cngge Learring.All Flights Reoorved
▪ Retro-aldol reaction cleaves a b-hydroxy carbonyl compound into two aldehydes or ketones ▪ C=O group is formed at C4 Catabolism of Glucose: Glycolysis

Catabolism of Glucose:Glycolysis Two classes of aldolases are used by organisms to catalyze the retro-aldol reaction Reaction catalyzed by class ll aldolases in fungi,algae,and some bacteria The catalyst functions by coordination of the fructose carbonyl group with Zn2+as Lewis acid Reaction catalyzed by class I aldolases in plant and animals Does not take place on the free ketone Fructose 1,6-bisphosphate undergoes reaction with the side-chain- NH2 group of a lysine residue on the aldolase to yield a protonated enzyme-bound imine called a Schiff base The iminium ion is a better electron acceptor than a ketone carbonyl group Retro-aldol reaction gives glyceraldehyde 3-phosphate and an enamine Enamine is protonated to give another iminium ion that is hydrolyzed to yield dihydroxyacetone phosphate
Two classes of aldolases are used by organisms to catalyze the retro-aldol reaction ▪ Reaction catalyzed by class II aldolases in fungi, algae, and some bacteria ▪ The catalyst functions by coordination of the fructose carbonyl group with Zn2+ as Lewis acid ▪ Reaction catalyzed by class I aldolases in plant and animals ▪ Does not take place on the free ketone ▪ Fructose 1,6-bisphosphate undergoes reaction with the side-chain – NH2 group of a lysine residue on the aldolase to yield a protonated enzyme-bound imine called a Schiff base ▪ The iminium ion is a better electron acceptor than a ketone carbonyl group ▪ Retro-aldol reaction gives glyceraldehyde 3-phosphate and an enamine ▪ Enamine is protonated to give another iminium ion that is hydrolyzed to yield dihydroxyacetone phosphate Catabolism of Glucose: Glycolysis
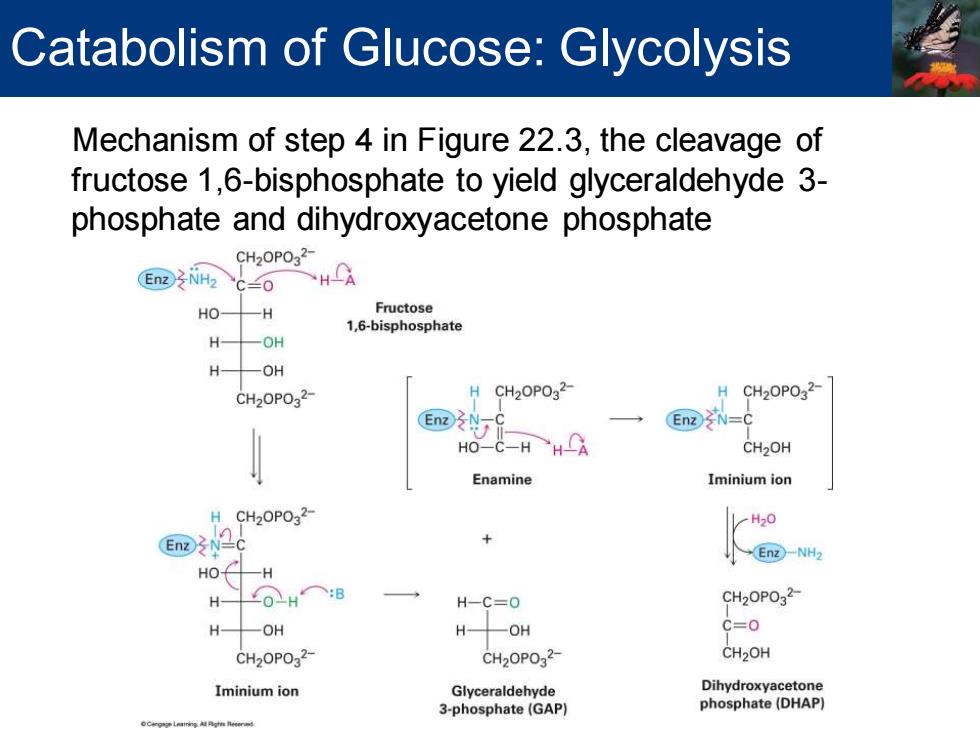
Catabolism of Glucose:Glycolysis Mechanism of step 4 in Figure 22.3,the cleavage of fructose 1,6-bisphosphate to yield glyceraldehyde 3- phosphate and dihydroxyacetone phosphate CH20P032 EnzNH2 “C兰0 HA HO- 一H Fructose 1,6-bisphosphate H —OH H -OH CH2OPO32- H CH20P032- CH2OPO32- EnzN=C HO C-HH CH2OH Enamine Iminium ion H CH2OPO32- En →En2-NH2 HO H HOH○:B H-C=0 CH2OPO2- H -OH H -OH C=0 CH2OPO32- CH20P032- CH2OH Iminium ion Glyceraldehyde Dihydroxyacetone 3-phosphate(GAP) phosphate(DHAP) d
Mechanism of step 4 in Figure 22.3, the cleavage of fructose 1,6-bisphosphate to yield glyceraldehyde 3- phosphate and dihydroxyacetone phosphate Catabolism of Glucose: Glycolysis
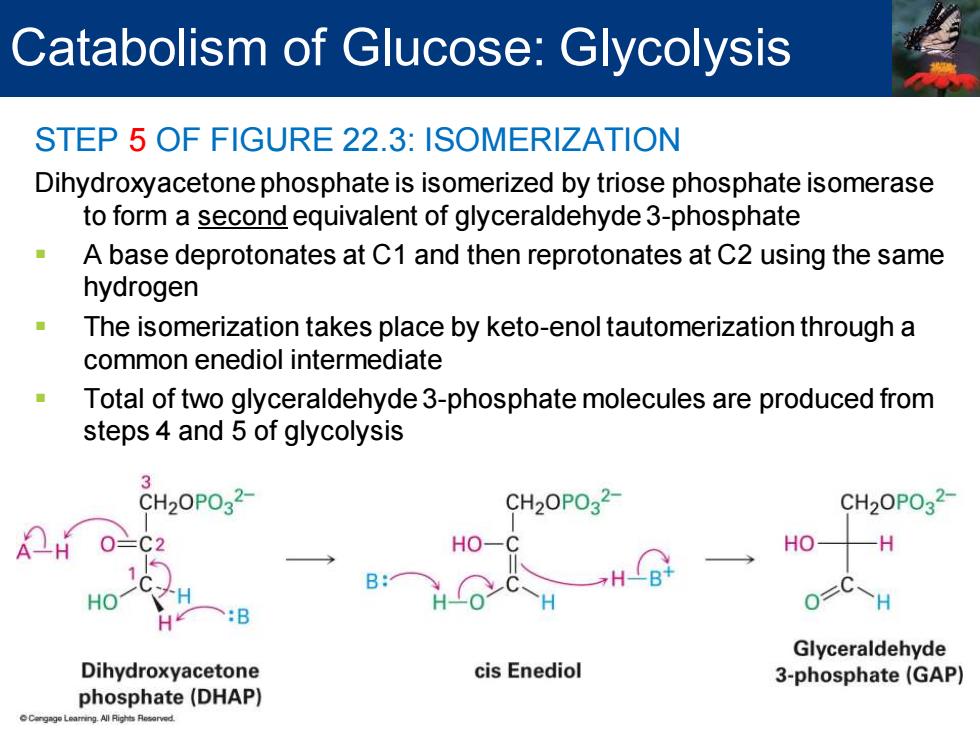
Catabolism of Glucose:Glycolysis STEP 5 OF FIGURE 22.3:ISOMERIZATION Dihydroxyacetone phosphate is isomerized by triose phosphate isomerase to form a second equivalent of glyceraldehyde 3-phosphate A base deprotonates at C1 and then reprotonates at C2 using the same hydrogen The isomerization takes place by keto-enol tautomerization through a common enediol intermediate Total of two glyceraldehyde 3-phosphate molecules are produced from steps 4 and 5 of glycolysis 3 2OPO32- CH2OPO32- CH20P032 HO- HO H B: OC~H :B Glyceraldehyde Dihydroxyacetone cis Enediol 3-phosphate(GAP) phosphate(DHAP)
STEP 5 OF FIGURE 22.3: ISOMERIZATION Dihydroxyacetone phosphate is isomerized by triose phosphate isomerase to form a second equivalent of glyceraldehyde 3-phosphate ▪ A base deprotonates at C1 and then reprotonates at C2 using the same hydrogen ▪ The isomerization takes place by keto-enol tautomerization through a common enediol intermediate ▪ Total of two glyceraldehyde 3-phosphate molecules are produced from steps 4 and 5 of glycolysis Catabolism of Glucose: Glycolysis
Catabolism of Glucose:Glycolysis STEPS 6-7 OF FIGURE 22.3:OXIDATION. PHOSPHORYATION.AND DEPHOSPHORYLATION Glyceraldehyde 3-phosphate is oxidized and phosphorylated in step 6 to give 1,3-bisphosphoglycerate Reaction is catalyzed by glyceraldehyde 3-phosphate dehydrogenase Nucleophilic addition of the-SH group of a cysteine residue in the enzyme to the aldehyde carbonyl group yields a hemithioacetal,the sulfur analog of a hemiacetal Oxidation of the hemithioacetal-OH group by NAD+yields a thioester Thioester reacts with phosphate ion in a nucleophilic acyl substitution step to give 1,3-bisphosphoglycerate
STEPS 6-7 OF FIGURE 22.3: OXIDATION, PHOSPHORYATION, AND DEPHOSPHORYLATION Glyceraldehyde 3-phosphate is oxidized and phosphorylated in step 6 to give 1,3-bisphosphoglycerate ▪ Reaction is catalyzed by glyceraldehyde 3-phosphate dehydrogenase ▪ Nucleophilic addition of the –SH group of a cysteine residue in the enzyme to the aldehyde carbonyl group yields a hemithioacetal, the sulfur analog of a hemiacetal ▪ Oxidation of the hemithioacetal –OH group by NAD+ yields a thioester ▪ Thioester reacts with phosphate ion in a nucleophilic acyl substitution step to give 1,3-bisphosphoglycerate Catabolism of Glucose: Glycolysis