
Elimination Reactions (Overview) El: CH,CH, CH,CH. CH.OH CH,CH, H- C-CH,CH: CH.OH -CH.CH CH::Br: CH :Br: H.C CH,CH CH,一O-H E2: CH,05 CHO-H CH,CH, H CHCH H C-CH,CH; Na'-OCH C= CH, CH,OH CH,CH :Br: Elimination reactions produce bonds. 。Also called (-HX). Chapter 6 16
Elimination Reactions (Overview) • Elimination reactions produce _______ bonds. • Also called _____________________ (-HX). Chapter 6 16
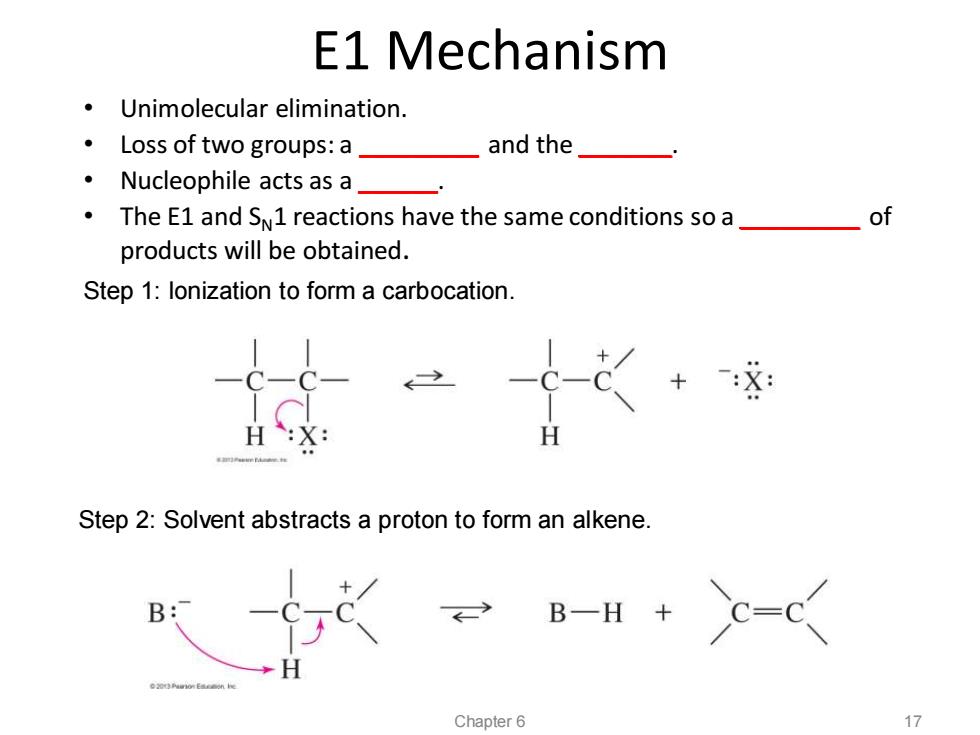
E1 Mechanism Unimolecular elimination. ·Loss of two groups:a and the Nucleophile acts as a_ The E1 and SN1 reactions have the same conditions so a_ of products will be obtained. Step 1:lonization to form a carbocation. Step 2:Solvent abstracts a proton to form an alkene. B: B-H Chapter 6 17
E1 Mechanism Step 1: Ionization to form a carbocation. Step 2: Solvent abstracts a proton to form an alkene. Chapter 6 17 • Unimolecular elimination. • Loss of two groups: a _________ and the _______. • Nucleophile acts as a ______. • The E1 and SN1 reactions have the same conditions so a _________ of products will be obtained

A Closer Look B: B一H H overlap .CH3 H.C CH3 -CH3 CH3 H empty p πbond Chapter 6 18
A Closer Look Chapter 6 18
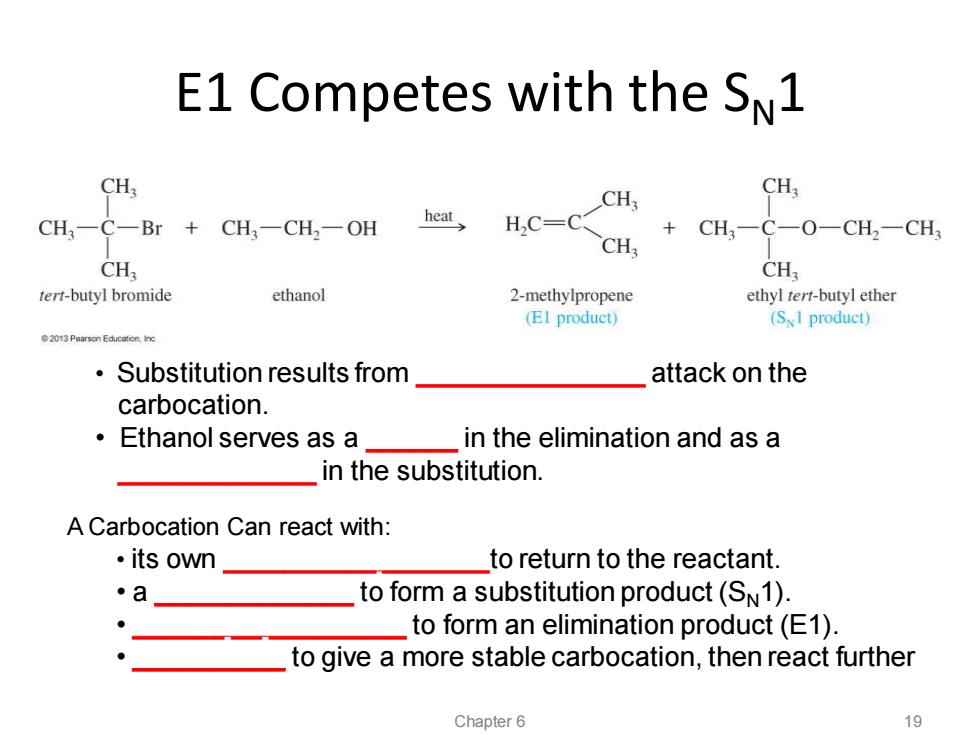
E1 Competes with the SN1 CH: CH CH: CH,一C-Br+CH-CH,-OH heat H,C=C +CH3一C一O-CH2一CH CH; CH CH tert-butyl bromide ethanol 2-methylpropene ethyl tert-butyl ether (EI product) (SI product) 2013 Pearson Education Ine Substitution results from attack on the carbocation. ·Ethanol serves as a in the elimination and as a in the substitution. A Carbocation Can react with: ·its own to return to the reactant 。a to form a substitution product(SN1). to form an elimination product(E1). to give a more stable carbocation,then react further Chapter 6 19
E1 Competes with the SN1 • Substitution results from _______________ attack on the carbocation. • Ethanol serves as a ______ in the elimination and as a _____________ in the substitution. Chapter 6 19 A Carbocation Can react with: • its own __________ _______to return to the reactant. • a _____________ to form a substitution product (SN1). • ______ __ _________ to form an elimination product (E1). • __________ to give a more stable carbocation, then react further
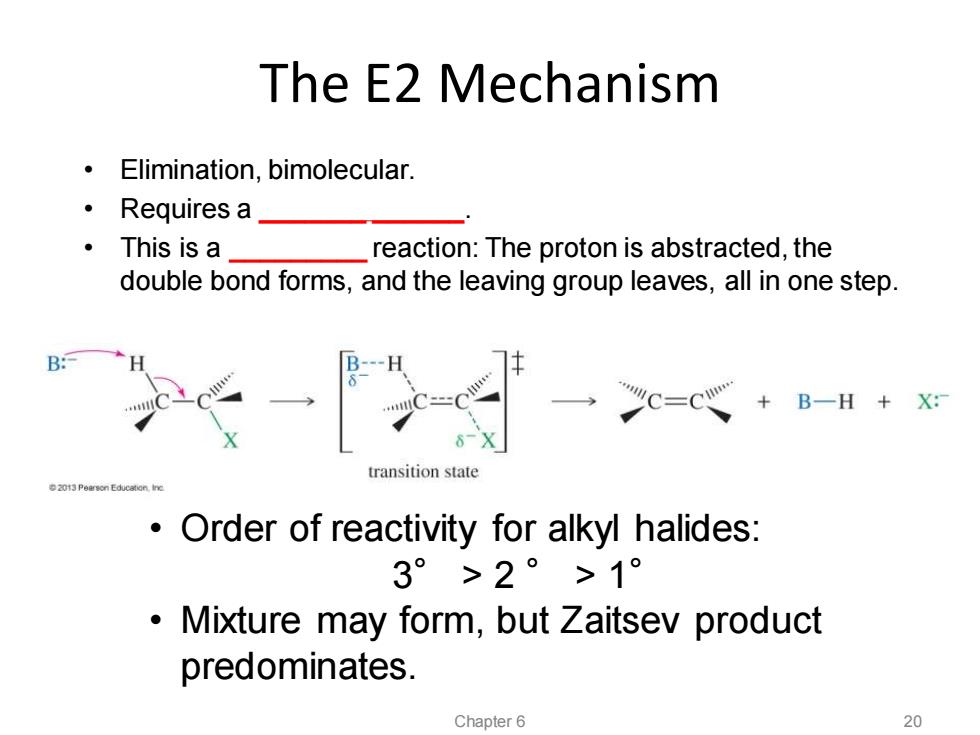
The E2 Mechanism Elimination,bimolecular. ·Requires a ·This is a _reaction:The proton is abstracted,the double bond forms,and the leaving group leaves,all in one step. B C=C心+B-H+X灯 transition state 2013 Pearson Education,Ine Order of reactivity for alkyl halides: 3°>2°>1° Mixture may form,but Zaitsev product predominates. Chapter 6 20
The E2 Mechanism • Order of reactivity for alkyl halides: 3° > 2 ° > 1° • Mixture may form, but Zaitsev product predominates. Chapter 6 20 • Elimination, bimolecular. • Requires a _______ ______. • This is a _________ reaction: The proton is abstracted, the double bond forms, and the leaving group leaves, all in one step