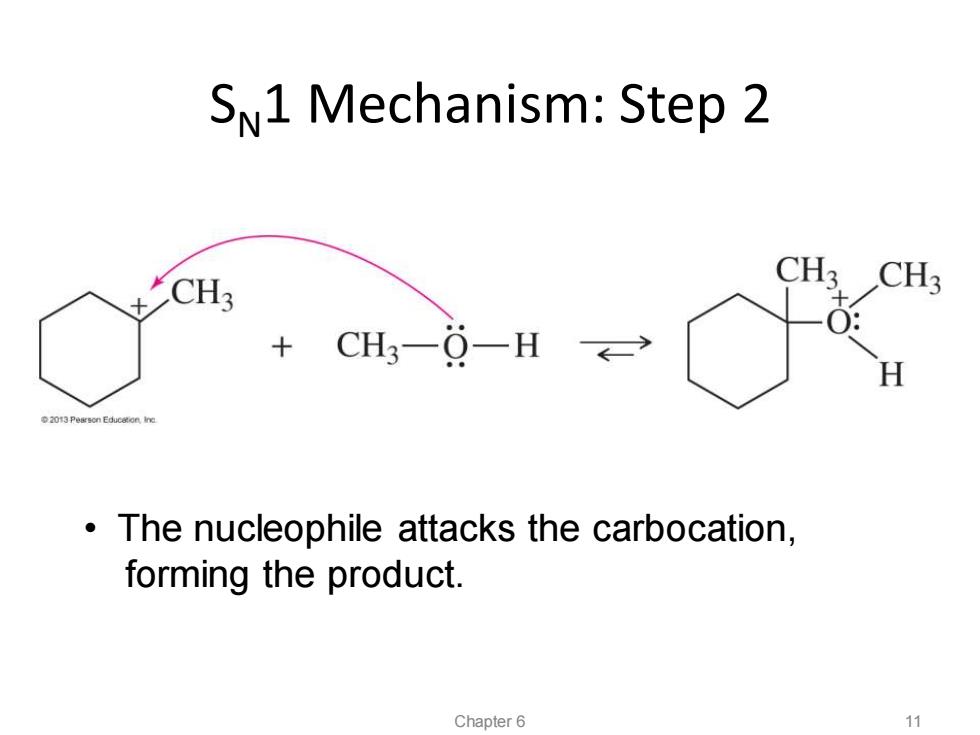
SN1 Mechanism:Step 2 CH3 CH3 CH3 CH3-0-H→ H 2013 Pearson Educeton Ine The nucleophile attacks the carbocation, forming the product. Chapter 6 11
SN1 Mechanism: Step 2 • The nucleophile attacks the carbocation, forming the product. Chapter 6 11

SN1 Mechanism:Step 3 CH3 CH3 CH3 CH3 + H +cH一9一H H product (protonated methanol) e203 Pearson Educe减nn If the nucleophile is neutral,a third step (deprotonation)is needed. Chapter 6 12
• If the nucleophile is neutral, a third step (deprotonation) is needed. SN1 Mechanism: Step 3 Chapter 6 12

Carbocation Stability vacant porbital weak overlap 6+ 8.CH3+CH3 carbocation stability: 3°>2°>1°>+CH3 H 6.CH3 inductive effect carbocation alkyl group hyperconjugation e2为Paon E线om探 Carbocations are stabilized by: effect Chapter 6 13
Carbocation Stability Carbocations are stabilized by: • _________ effect • _________________. Chapter 6 13
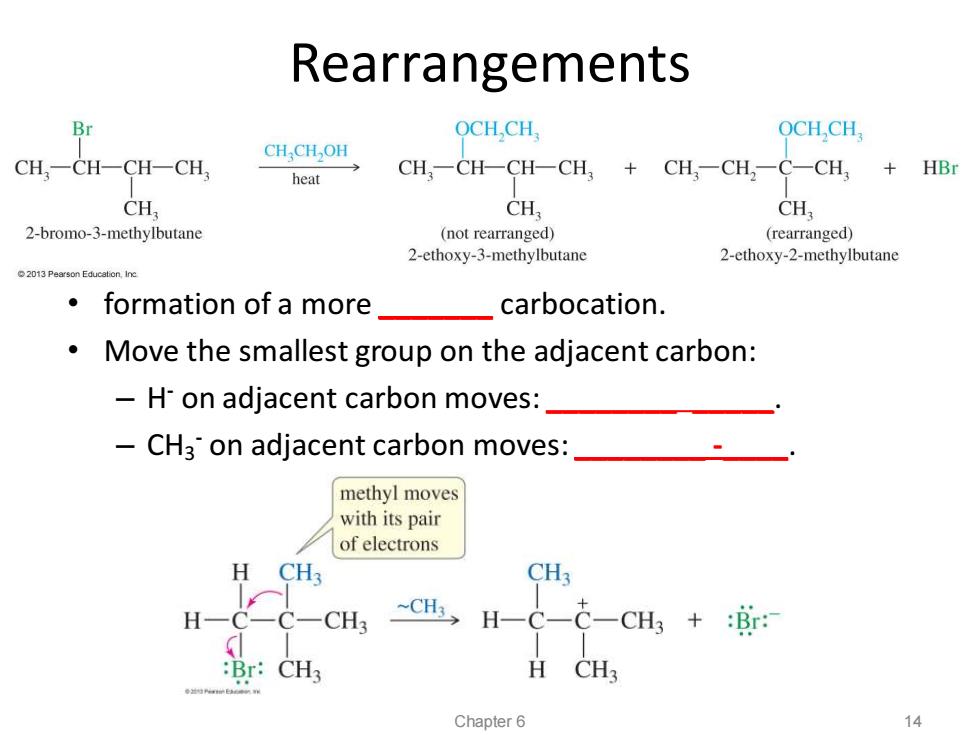
Rearrangements Br OCH,CH OCHCH, CHCH OH CH,-CH-CH-CH, heat CH,-CH-CH-CH,CH,-CH,-C-CH3 HBr CH CH CH, 2-bromo-3-methylbutane (not rearranged) (rearranged) 2-ethoxy-3-methylbutane 2-ethoxy-2-methylbutane 2013 Pearson Educalion,Inc 。formation of a more_ carbocation. Move the smallest group on the adjacent carbon: H-on adjacent carbon moves: -CH3 on adjacent carbon moves: methyl moves with its pair of electrons H CH3 CH3 H C-C- CH3 -CH3, H-C-C-CH3 +:r: :Br:CH3 H CH3 Chapter 6 14
Rearrangements • formation of a more _______ carbocation. • Move the smallest group on the adjacent carbon: – H - on adjacent carbon moves: ________ _____. – CH3 - on adjacent carbon moves: ________ -____. Chapter 6 14
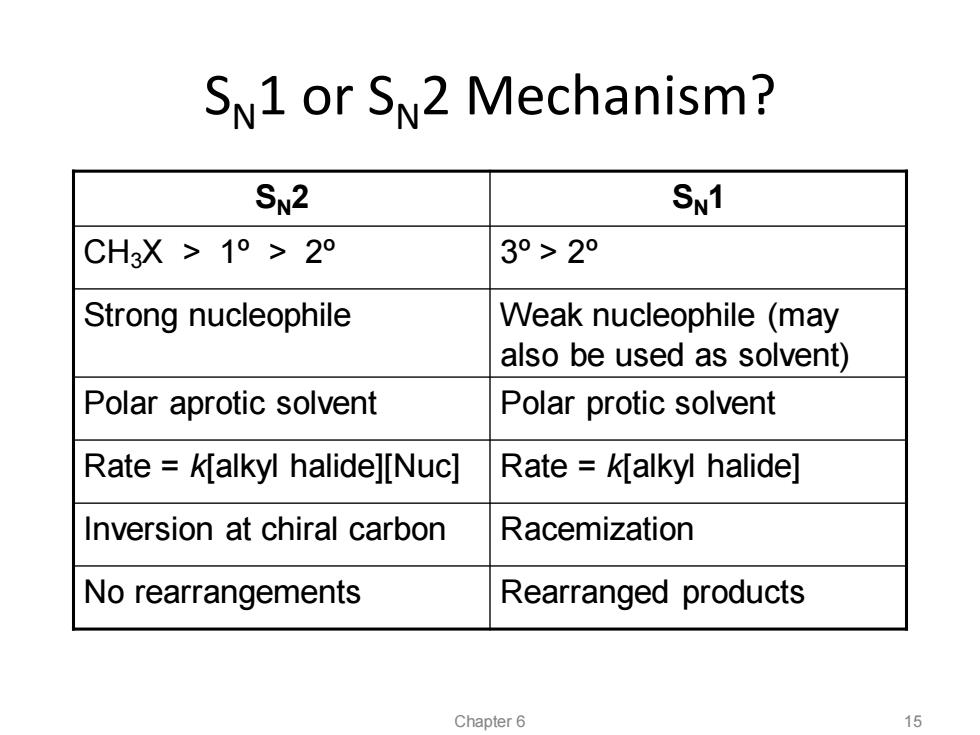
SN1 or SN2 Mechanism? SN2 SN1 CH3X>1°>2° 3°>2° Strong nucleophile Weak nucleophile (may also be used as solvent) Polar aprotic solvent Polar protic solvent Rate k[alkyl halide][Nuc] Rate k[alkyl halide] Inversion at chiral carbon Racemization No rearrangements Rearranged products Chapter 6 15
SN1 or SN2 Mechanism? SN2 SN1 CH3X > 1º > 2º 3º> 2º Strong nucleophile Weak nucleophile (may also be used as solvent) Polar aprotic solvent Polar protic solvent Rate = k[alkyl halide][Nuc] Rate = k[alkyl halide] Inversion at chiral carbon Racemization No rearrangements Rearranged products Chapter 6 15