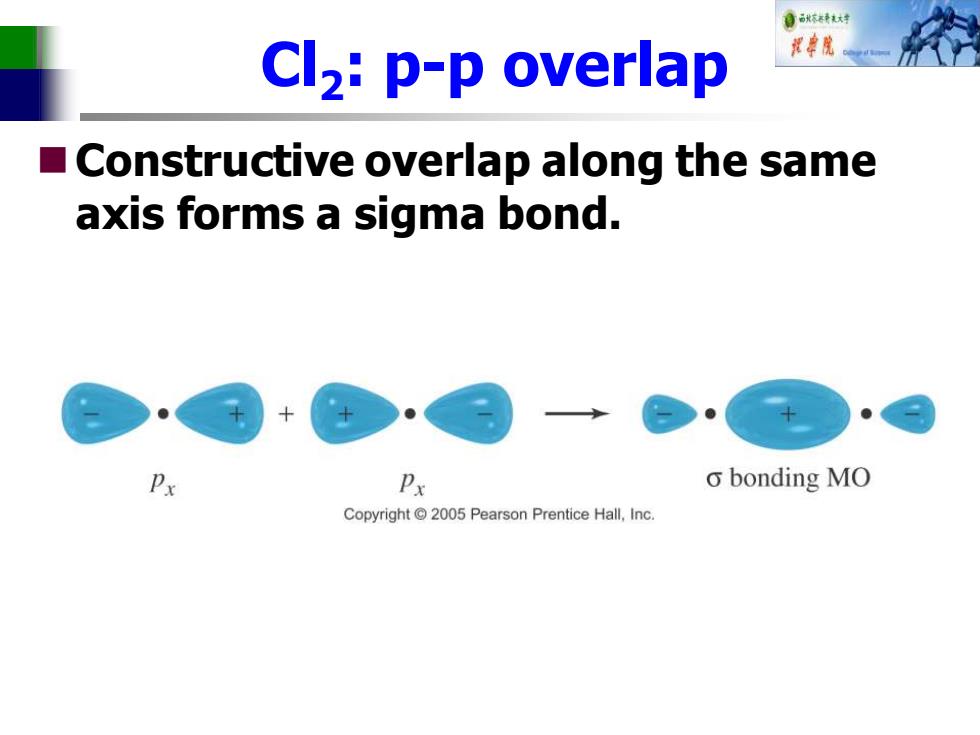
自秋不转大对 Cl2:p-p overlap 中院 Constructive overlap along the same axis forms a sigma bond. Px o bonding MO Copyright 2005 Pearson Prentice Hall,Inc
Cl2 : p-p overlap ◼Constructive overlap along the same axis forms a sigma bond
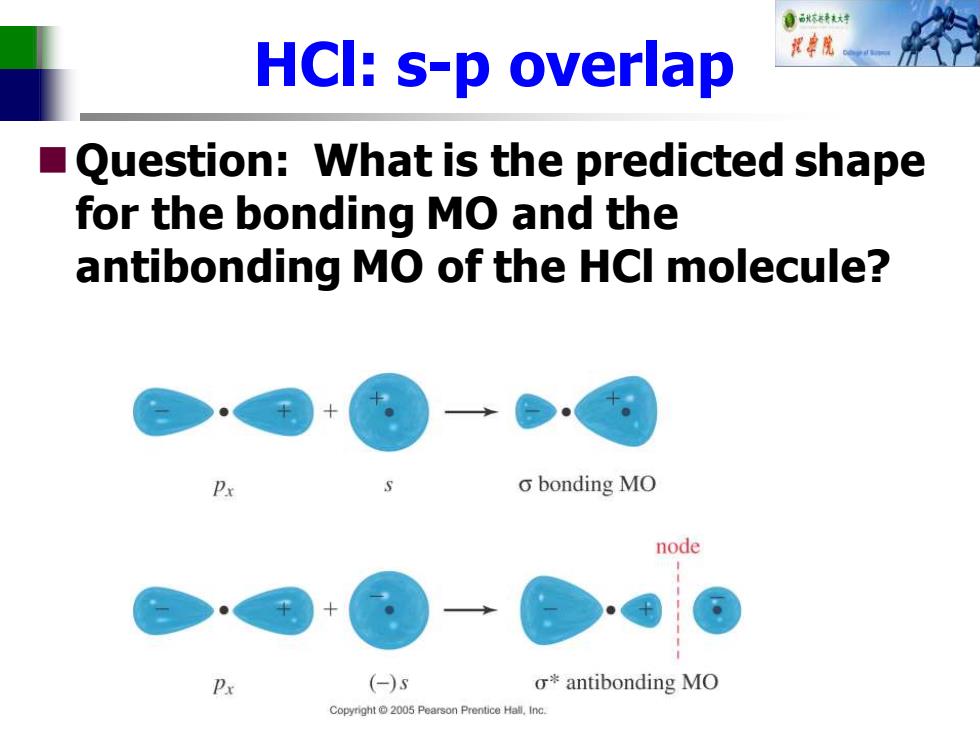
自秋不特大对 HCI:s-p overlap ■( Question:What is the predicted shape for the bonding MO and the antibonding MO of the HCI molecule? o bonding MO node Px (-)s o*antibonding MO Copyright 2005 Pearson Prentice Hall.Inc
HCl: s-p overlap ◼Question: What is the predicted shape for the bonding MO and the antibonding MO of the HCl molecule?
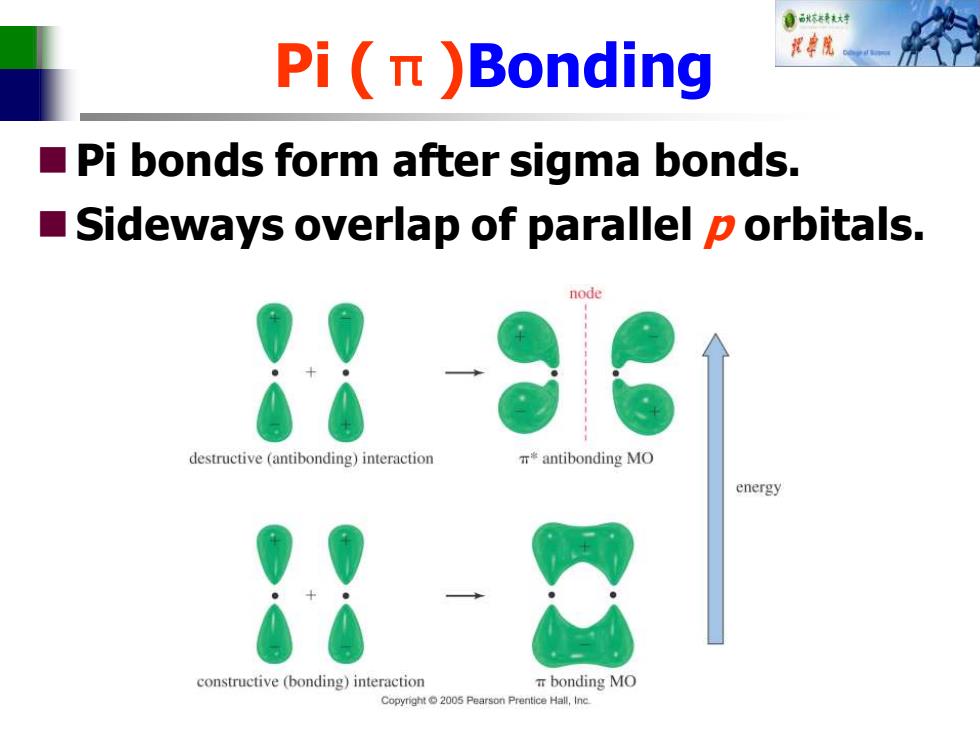
自标特大对 Pi(n )Bonding ■ Pi bonds form after sigma bonds. ■ Sideways overlap of parallel p orbitals. node destructive(antibonding)interaction r◆antibonding MO energy constructive (bonding)interaction T bonding MO Copyright2005 Pearson Prentice Hall,Inc
Pi (π)Bonding ◼Pi bonds form after sigma bonds. ◼Sideways overlap of parallel p orbitals

自秋不特大对 Multiple Bonds A double bond (2 pairs of shared electrons)consists of a sigma bond and a pi bond. A triple bond (3 pairs of shared electrons)consists of a sigma bond and two pi bonds. half of m bond H o bond H H half of bond
Multiple Bonds ◼A double bond (2 pairs of shared electrons) consists of a sigma bond and a pi bond. ◼A triple bond (3 pairs of shared electrons) consists of a sigma bond and two pi bonds
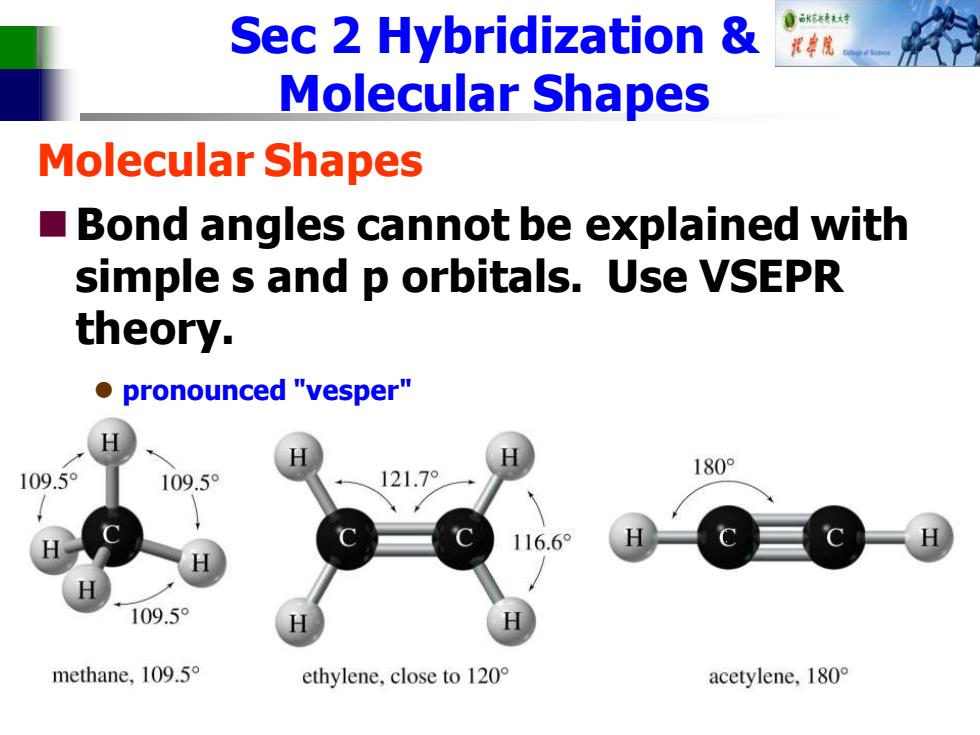
Sec 2 Hybridization 自秋韩大对 Molecular Shapes Molecular Shapes ■ Bond angles cannot be explained with simple s and p orbitals.Use VSEPR theory. ●pronounced"vesper" 109.5° 121.7° 180° 109.5° 116.6° 109.5° methane,109.5 ethylene,close to 120 acetylene,l8o°
Sec 2 Hybridization & Molecular Shapes Molecular Shapes ◼Bond angles cannot be explained with simple s and p orbitals. Use VSEPR theory. ⚫ pronounced "vesper" ⚫ Valence Shell Electron Pair Repulsion Theory