
自标4花对 Bonding Region ■ Electrons are close to both nuclei. bonding region ● electrons in this region nucleus 1 nucleus 2 attract both nuclei and mask the positive charges from repelling each other Copyright2005 Pearson Prentice Hall,Inc
Bonding Region ◼Electrons are close to both nuclei
Sigma(o)Bonding ■ Electron density lies between the nuclei. A bond may be formed by s-s,p-p,s-p, or hybridized orbital overlaps. ■ The bonding MO is lower in energy than the original atomic orbitals. The antibonding MO is higher in energy than the atomic orbitals
Sigma(σ) Bonding ◼Electron density lies between the nuclei. ◼A bond may be formed by s-s, p-p, s-p, or hybridized orbital overlaps. ◼The bonding MO is lower in energy than the original atomic orbitals. ◼The antibonding MO is higher in energy than the atomic orbitals
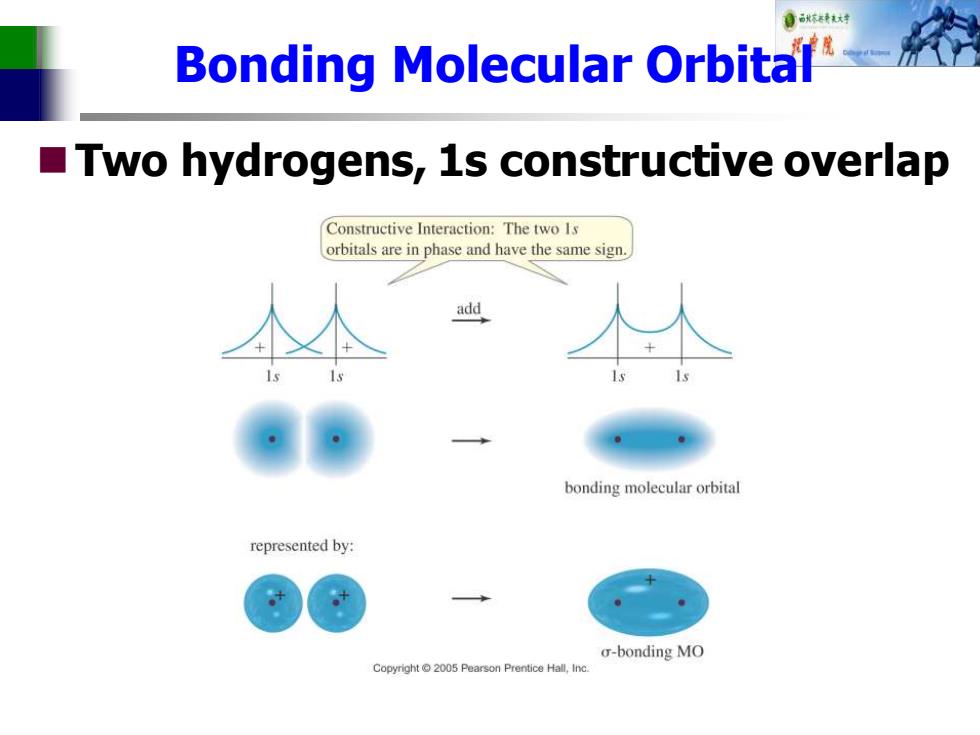
Bonding Molecular Orbital Two hydrogens,1s constructive overlap Constructive Interaction:The two Is orbitals are in phase and have the same sign. add bonding molecular orbital represented by: o-bonding MO Copyright2005 Pearson Prentice Hall,Inc
Bonding Molecular Orbital ◼Two hydrogens, 1s constructive overlap

自秋对 Anti-Bonding Molecular Orbital Two hydrogens,destructive overlap. Destructive interaction:The two Is orbitals are out of phase. anubonding molecular orbital represented by: node aantibonding MO Copyright2005 Pearson Prentice Hall,Inc
Anti-Bonding Molecular Orbital ◼Two hydrogens, destructive overlap

自秋转大材 花单院 H,:s-s overlap node antibonding 0* energy atomic orbital atomic orbital bonding molecular orbital Copyright 2005 Pearson Prentice Hall,Inc
H2 : s-s overlap