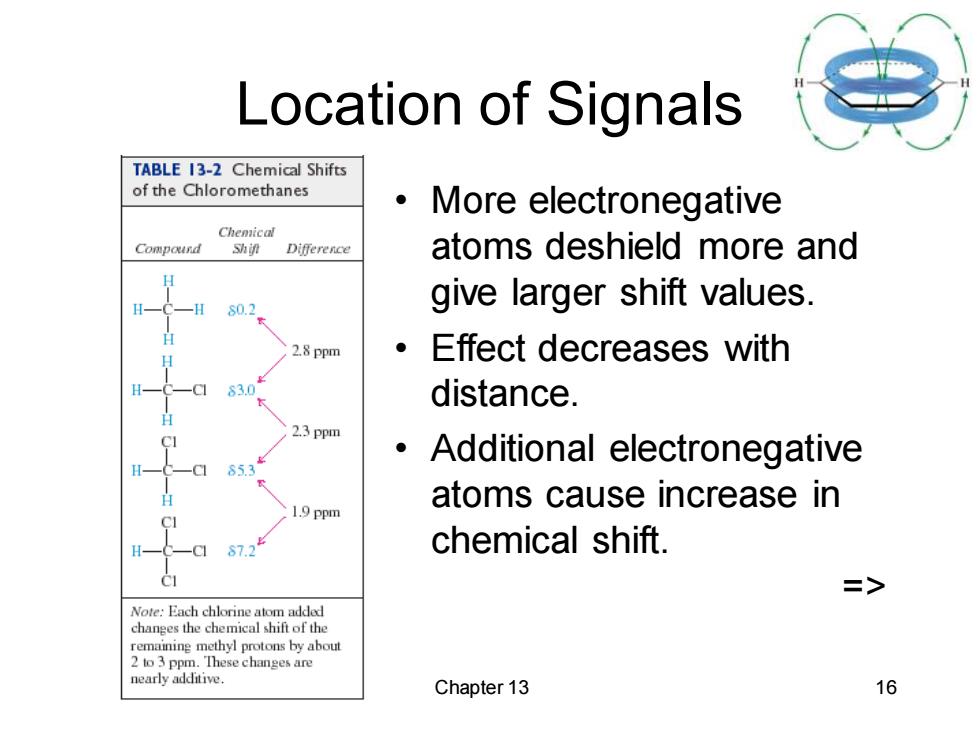
Location of Signals TABLE 13-2 Chemical Shifts of the Chloromethanes More electronegative Chemical Compound Shif Difference atoms deshield more and S0.2 give larger shift values. 2.8 ppm Effect decreases with 53n distance. 2.3 ppm Additional electronegative 65.3 atoms cause increase in 1.9 ppm 87.2 chemical shift. => Note:Each chlorine atom added changes the chemical shift of the remaining methyl protons by about 2 to 3 ppm.These changes are nearly additive. Chapter 13 16
Chapter 13 16 Location of Signals • More electronegative atoms deshield more and give larger shift values. • Effect decreases with distance. • Additional electronegative atoms cause increase in chemical shift. =>
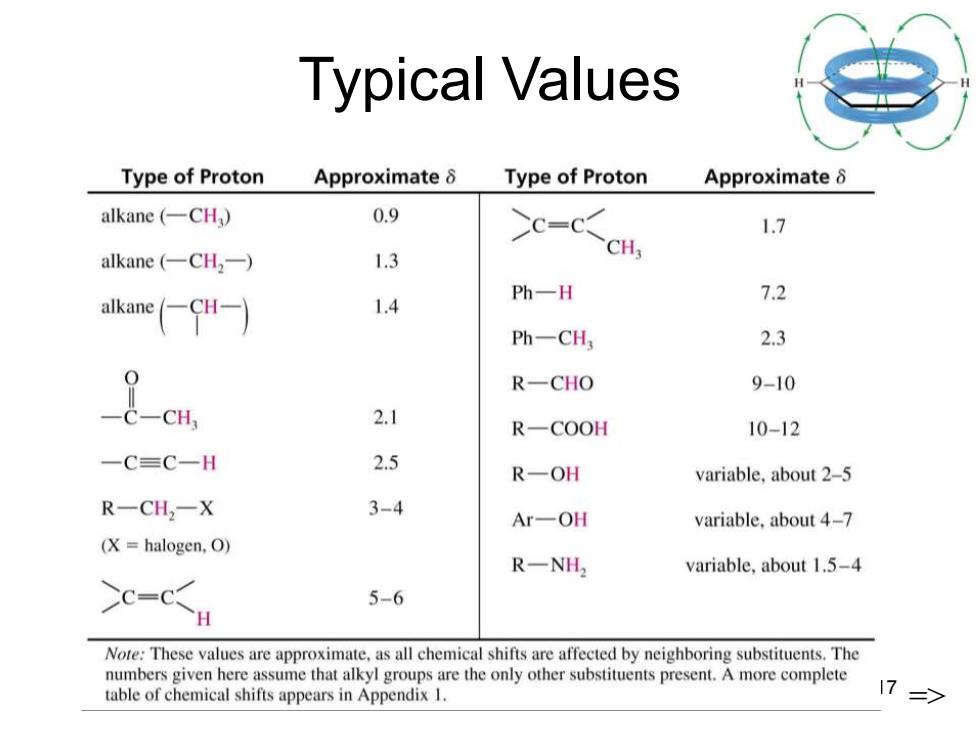
Typical Values Type of Proton Approximate 8 Type of Proton Approximate 8 alkane(一CH) 0.9 c-c<ct 1.7 alkane(-CH2-) 1.3 akae(←CH-) 1.4 Ph一H 7.2 Ph一CH 2.3 R-CHO 9-10 2.1 R一COOH 10-12 一C=C一H 2.5 R-OH variable,about 2-5 R一CH2一X 3-4 Ar-OH variable,about 4-7 (X halogen,O) R一NH2 variable,about 1.5-4 >c-c 5-6 H Note:These values are approximate,as all chemical shifts are affected by neighboring substituents.The numbers given here assume that alkyl groups are the only other substituents present.A more complete table of chemical shifts appears in Appendix 1. 17
Chapter 13 17 Typical Values =>
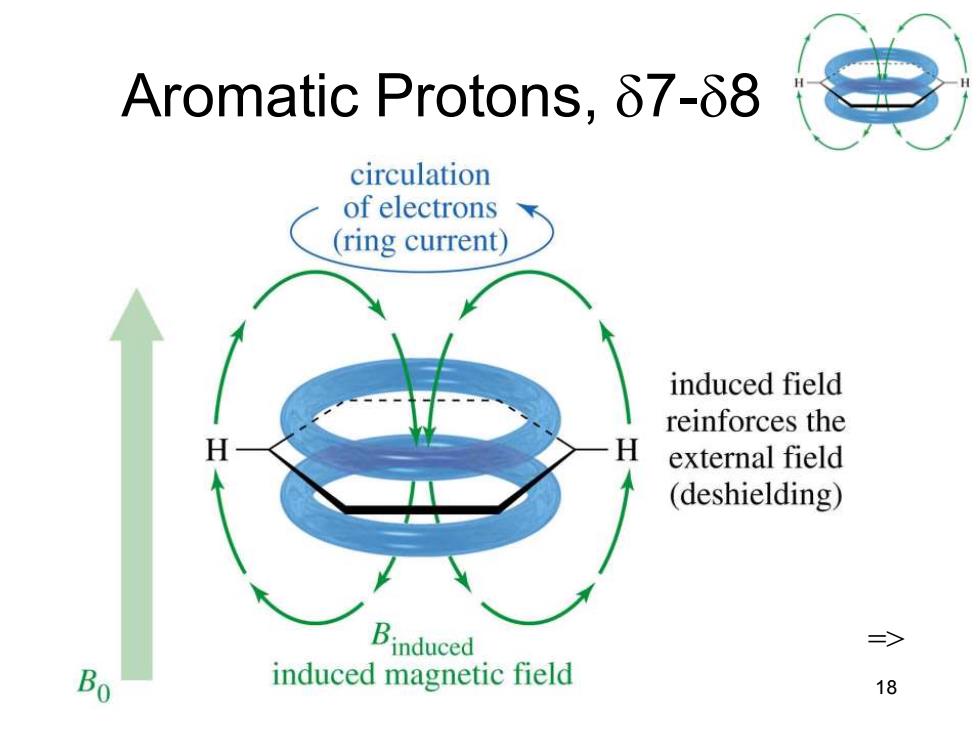
Aromatic Protons,87-88 circulation of electrons (ring current) induced field reinforces the H external field (deshielding) Binduced => Bo induced magnetic field 18
Chapter 13 18 Aromatic Protons, 7-8 =>
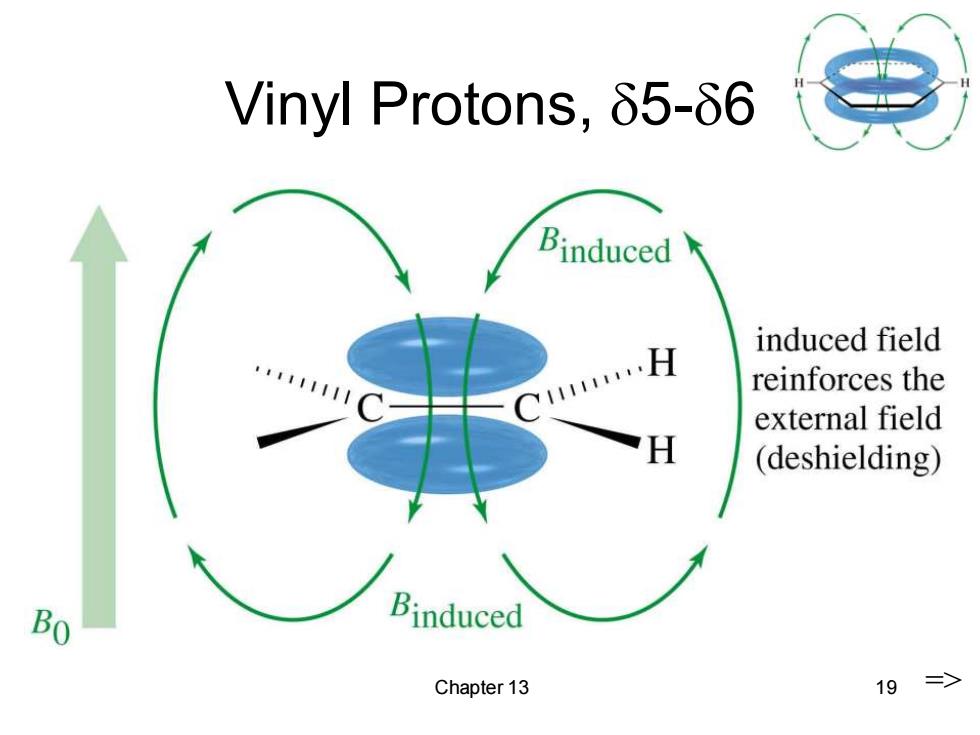
Vinyl Protons,δ5-66 Binduced induced field …H reinforces the external field H (deshielding) Bo Binduced Chapter 13 19 =>
Chapter 13 19 Vinyl Protons, 5-6 =>