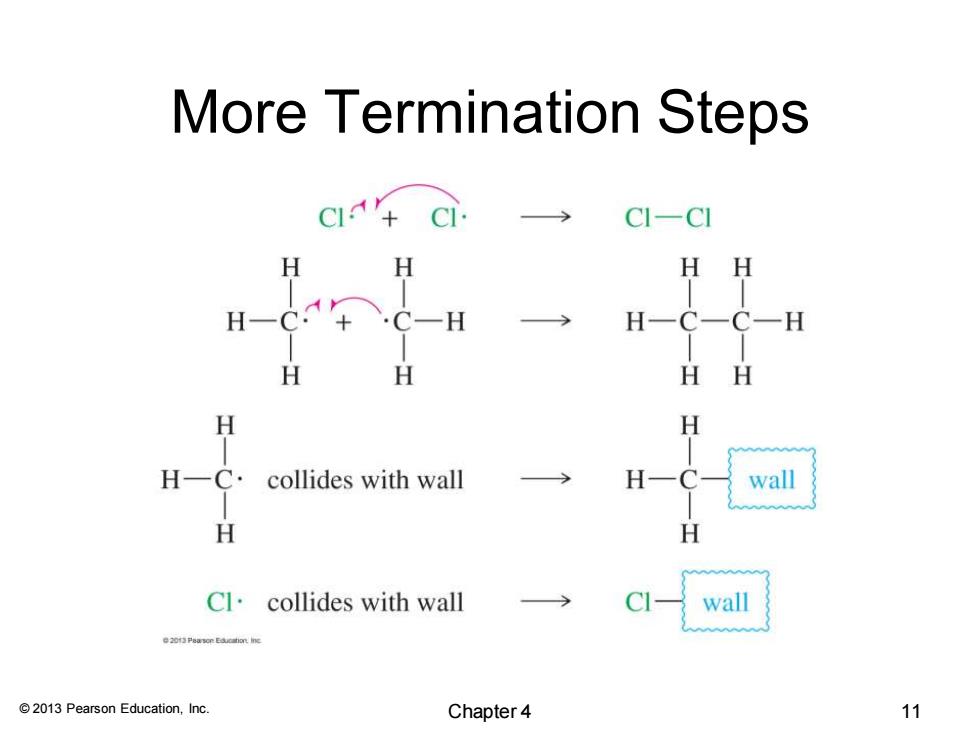
More Termination Steps C1+ CIC】 HH 一H H一C一C一H H H HH H H H C collides with wall wall H H Cl·collides with wall wall e23Per线m 2013 Pearson Education,Inc. Chapter 4 11
© 2013 Pearson Education, Inc. More Termination Steps Chapter 4 11

HINT Initiation steps generally create new free radicals. Propagation steps usually combine a free radical and a reactant to give a product and another free radical. Termination steps generally decrease the number of free radicals. 2013 Pearson Education,Inc. Chapter4 12
© 2013 Pearson Education, Inc. Initiation steps generally create new free radicals. Propagation steps usually combine a free radical and a reactant to give a product and another free radical. Termination steps generally decrease the number of free radicals. Chapter 4 12
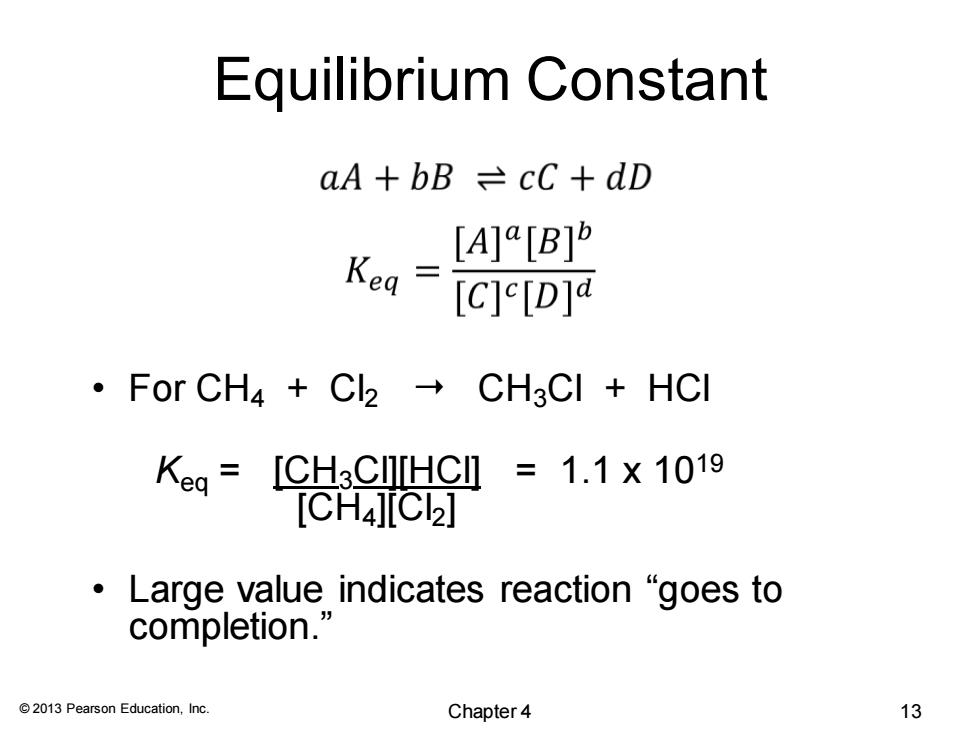
Equilibrium Constant aA+bB cC+dD [A]R[B] Kea [C]e[D]a ·For CH4+C2→CH3CI+HCI [CH3 CITTHC=1.1×1019 [CH4][C2] ·Large value indicates reaction“goes to completion.' 2013 Pearson Education,Inc. Chapter4 13
© 2013 Pearson Education, Inc. Equilibrium Constant • For CH4 + Cl2 CH3Cl + HCl Keq = [CH3Cl][HCl] = 1.1 x 1019 [CH4][Cl2] • Large value indicates reaction “goes to completion.” Chapter 4 13

Free Energy Change AG =(energy of products)-(energy of reactants) AG is the amount of energy available to do work. ·A reaction with a negative△G is favorable and spontaneous. △G°=-RT(In Kea)=-2.303RT(og1oKa) Keg =e-AG'/RT where R 8.314 J/K-mol,T=temperature in kelvins,e 2.718(base of natural log). 2013 Pearson Education,Inc. Chapter4 14
© 2013 Pearson Education, Inc. Free Energy Change ▪ DG = (energy of products) - (energy of reactants) ▪ DG is the amount of energy available to do work. • A reaction with a negative DG is favorable and spontaneous. DGo = -RT(lnKeq) = -2.303RT(log10Keq) where R = 8.314 J/K-mol, T = temperature in kelvins, e = 2.718 (base of natural log). Chapter 4 14
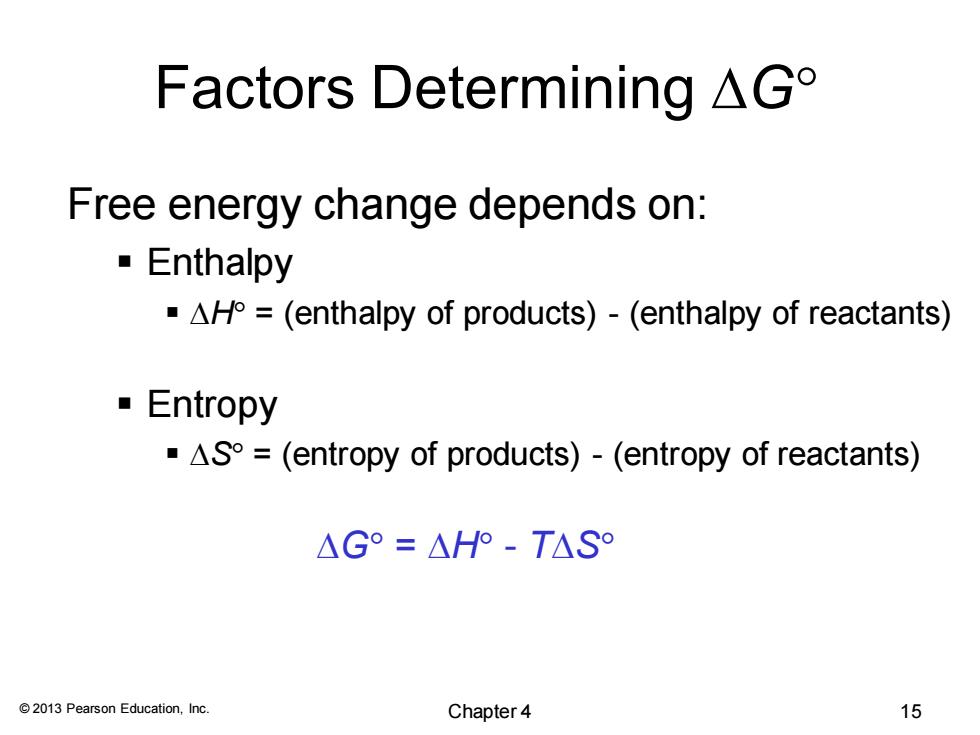
Factors Determining△G° Free energy change depends on: Enthalpy AH=(enthalpy of products)-(enthalpy of reactants) Entropy -AS=(entropy of products)-(entropy of reactants) △G°=△HP-T△S° 2013 Pearson Education,Inc. Chapter4 15
© 2013 Pearson Education, Inc. Factors Determining DG Free energy change depends on: ▪ Enthalpy ▪ DH = (enthalpy of products) - (enthalpy of reactants) ▪ Entropy ▪ DS = (entropy of products) - (entropy of reactants) DG = DH - TDS Chapter 4 15