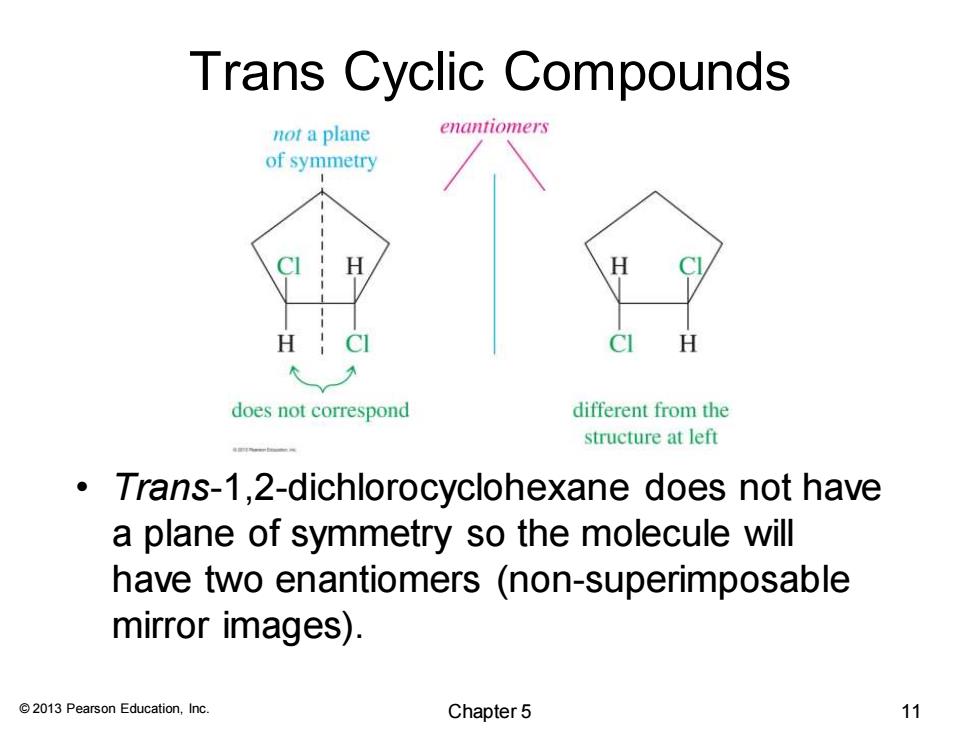
Trans Cyclic Compounds not a plane enantiomers of symmetry CI H does not correspond different from the structure at left Trans-1,2-dichlorocyclohexane does not have a plane of symmetry so the molecule will have two enantiomers (non-superimposable mirror images). 2013 Pearson Education,Inc. Chapter 5 11
© 2013 Pearson Education, Inc. Trans Cyclic Compounds • Trans-1,2-dichlorocyclohexane does not have a plane of symmetry so the molecule will have two enantiomers (non-superimposable mirror images). Chapter 5 11
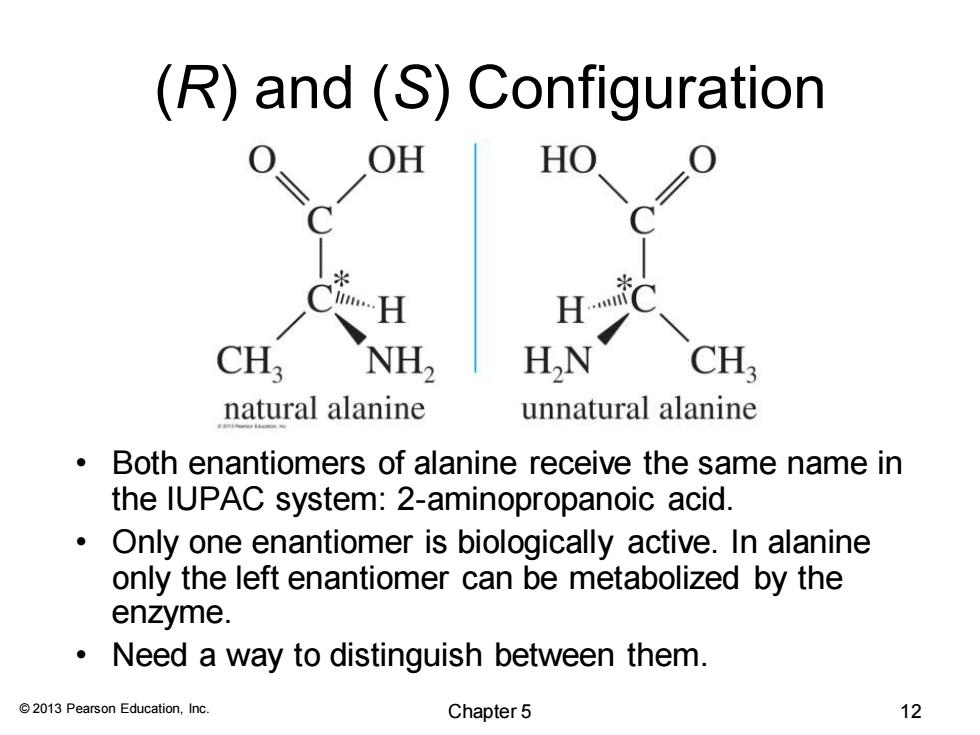
(R)and (S)Configuration OH HO CH; NH2 HN CH; natural alanine unnatural alanine Both enantiomers of alanine receive the same name in the IUPAC system:2-aminopropanoic acid. Only one enantiomer is biologically active.In alanine only the left enantiomer can be metabolized by the enzyme. Need a way to distinguish between them. 2013 Pearson Education,Inc. Chapter 5 12
© 2013 Pearson Education, Inc. (R) and (S) Configuration • Both enantiomers of alanine receive the same name in the IUPAC system: 2-aminopropanoic acid. • Only one enantiomer is biologically active. In alanine only the left enantiomer can be metabolized by the enzyme. • Need a way to distinguish between them. Chapter 5 12

Cahn-Ingold-Prelog Convention Enantiomers have different spatial arrangements (configurations)of the 4 groups attached to the asymmetric carbon. Each asymmetric carbon atom is assigned a letter (R)or (S)based on its three-dimensional configuration. Cahn-Ingold-Prelog convention is the most widely accepted system for naming the configurations of chirality centers. 2013 Pearson Education,Inc. Chapter5 13
© 2013 Pearson Education, Inc. Cahn–Ingold–Prelog Convention • Enantiomers have different spatial arrangements (configurations) of the 4 groups attached to the asymmetric carbon. • Each asymmetric carbon atom is assigned a letter (R) or (S) based on its three-dimensional configuration. • Cahn–Ingold–Prelog convention is the most widely accepted system for naming the configurations of chirality centers. Chapter 5 13
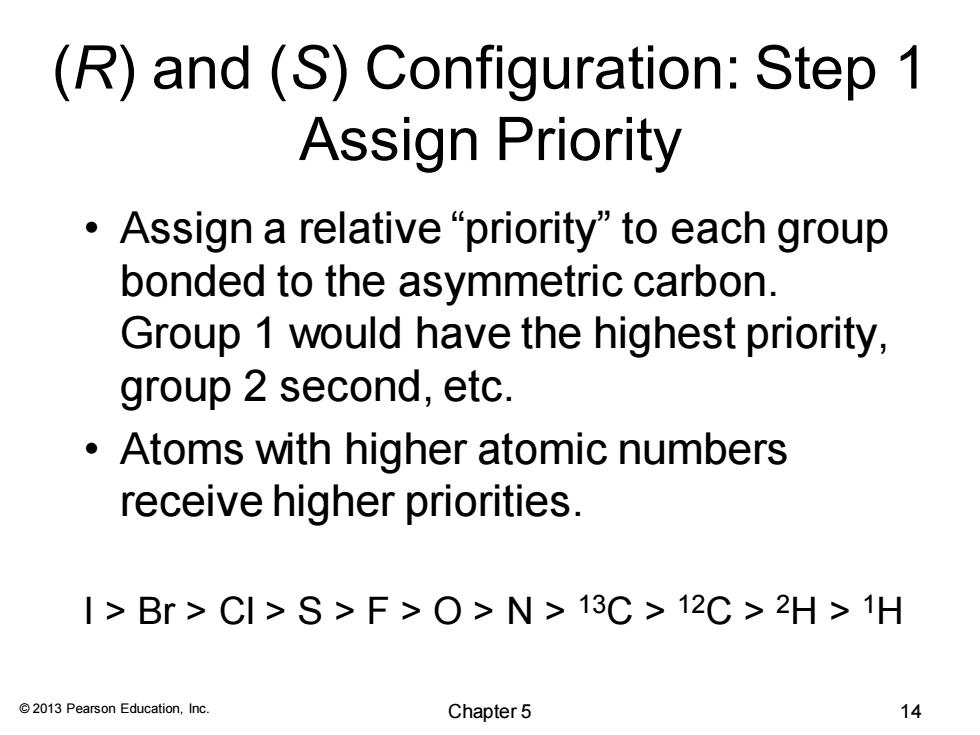
(R)and (S)Configuration:Step 1 Assign Priority ·Assign a relative“priority"to each group bonded to the asymmetric carbon. Group 1 would have the highest priority, group 2 second,etc. Atoms with higher atomic numbers receive higher priorities. I>Br>CI>S>F>O>N>13C>12C>2H>1H 2013 Pearson Education,Inc. Chapter5 14
© 2013 Pearson Education, Inc. (R) and (S) Configuration: Step 1 Assign Priority • Assign a relative “priority” to each group bonded to the asymmetric carbon. Group 1 would have the highest priority, group 2 second, etc. • Atoms with higher atomic numbers receive higher priorities. I > Br > Cl > S > F > O > N > 13C > 12C > 2H > 1H Chapter 5 14

Assign Priorities CH H NH2 Atomic number:F N C H 2013 Pearson Education,Inc. Chapter5 15
© 2013 Pearson Education, Inc. Assign Priorities Atomic number: F > N > C > H Chapter 5 15