
Basicity of Amines CH2CH3 Lone pair of electrons on nitrogen can accept a proton from an acid Aqueous solutions are basic to litmus. ·Ammonia pK.=4.74 Alkyl amines are usually stronger bases than ammonia.Increasing the number of alkyl groups decreases solvation of ion,so 2°and3°amines are similar to1°amines in basicity. => Chapter 19 16
Chapter 19 16 Basicity of Amines • Lone pair of electrons on nitrogen can accept a proton from an acid • Aqueous solutions are basic to litmus. • Ammonia pKb = 4.74 • Alkyl amines are usually stronger bases than ammonia. Increasing the number of alkyl groups decreases solvation of ion, so 2 and 3 amines are similar to 1 amines in basicity. =>
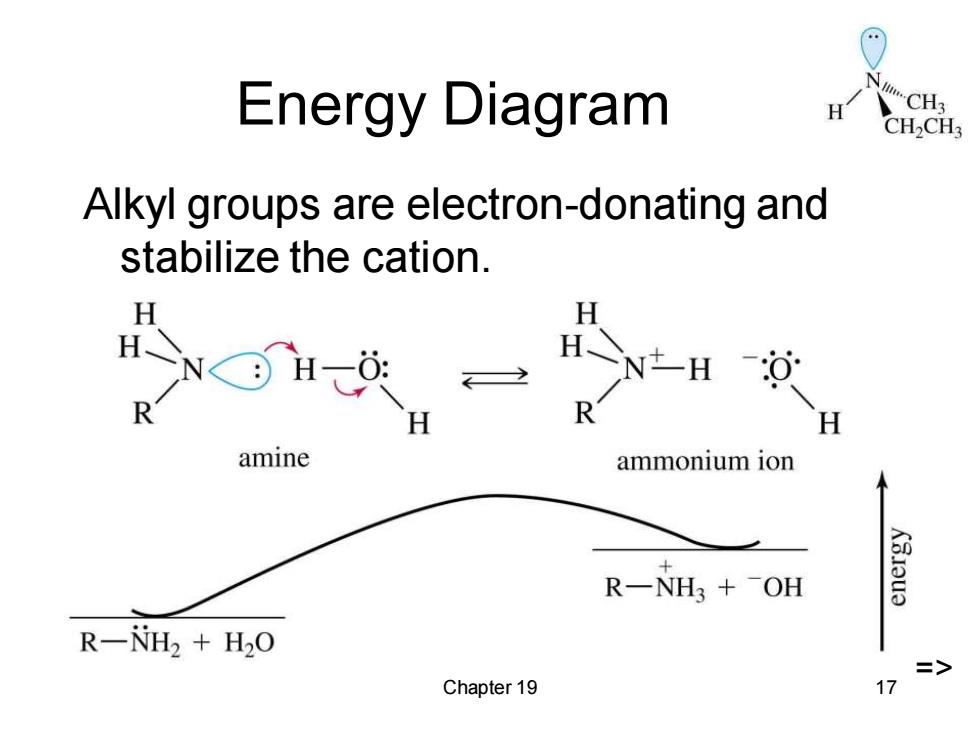
Energy Diagram H CH2CH3 Alkyl groups are electron-donating and stabilize the cation. H H H H amine ammonium ion R一NH3+-OH R-NH2 H2O Chapter 19 17
Chapter 19 17 Energy Diagram Alkyl groups are electron-donating and stabilize the cation. =>

Resonance Effects CH2CH3 Any delocalization of the electron pair weakens the base. H+ aniline anilinium ion stabilized by overlap with the ring no overlap is possible aliphatic amine R一NH2+H R-NH3NH3 stabilized by more endothermic overlap ○-less basic > ○-NH2+H aromatic amine 18
Chapter 19 18 Resonance Effects Any delocalization of the electron pair weakens the base. =>

Hybridization Effects H CH2CH3 Electrons are held more tightly in orbitals with more s character,so those compounds are weaker bases. H N sphybridized sp2hybridized (more basic) (less basic) pyridine,pKp=8.75 piperidine,pKp=2.88 => Chapter 19 19
Chapter 19 19 Hybridization Effects Electrons are held more tightly in orbitals with more s character, so those compounds are weaker bases. =>