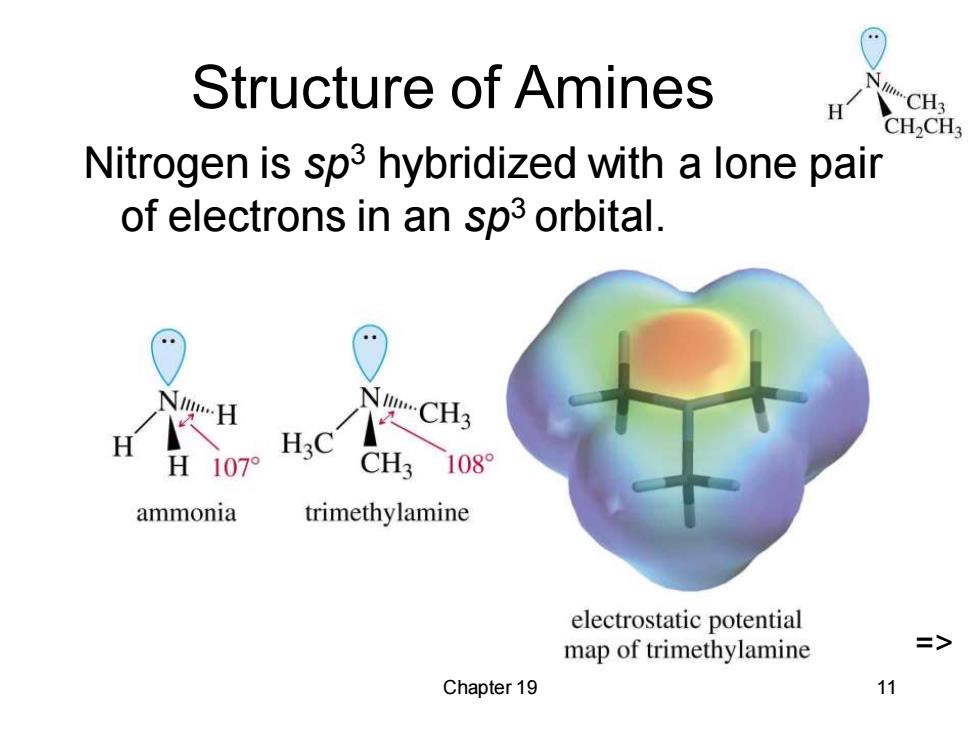
Structure of Amines H CH3 CH2CH3 Nitrogen is sp3 hybridized with a lone pair of electrons in an sp3 orbital. NCH3 H3C H107 CH3 108 ammonia trimethylamine electrostatic potential map of trimethylamine Chapter 19 11
Chapter 19 11 Structure of Amines Nitrogen is sp3 hybridized with a lone pair of electrons in an sp3 orbital. =>
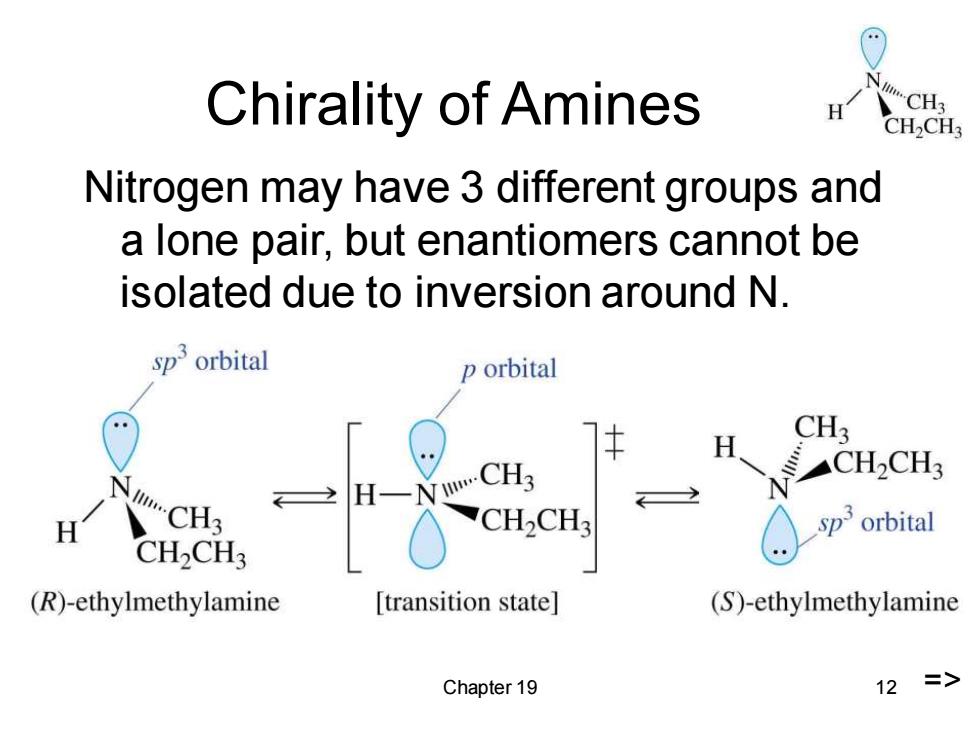
Chirality of Amines CH2CH3 Nitrogen may have 3 different groups and a lone pair,but enantiomers cannot be isolated due to inversion around N. sporbital p orbital H CH3 CH2CH3 2H一 H CH2CH3 sporbital CH2CH3 (R)-ethylmethylamine [transition state] (S)-ethylmethylamine Chapter 19 12 =>
Chapter 19 12 Chirality of Amines Nitrogen may have 3 different groups and a lone pair, but enantiomers cannot be isolated due to inversion around N. =>
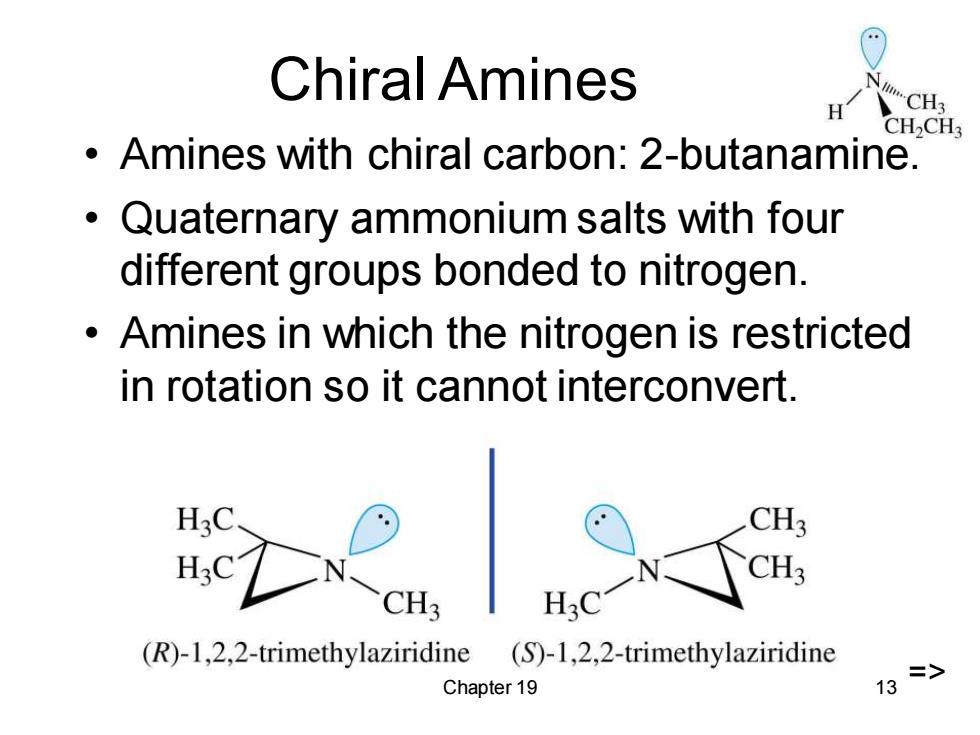
Chiral Amines H CH3 CH2CH Amines with chiral carbon:2-butanamine. Quaternary ammonium salts with four different groups bonded to nitrogen. Amines in which the nitrogen is restricted in rotation so it cannot interconvert. CH3 CH3 H.C (R)-1,2,2-trimethylaziridine (S)-1,2,2-trimethylaziridine Chapter 19 => 13
Chapter 19 13 Chiral Amines • Amines with chiral carbon: 2-butanamine. • Quaternary ammonium salts with four different groups bonded to nitrogen. • Amines in which the nitrogen is restricted in rotation so it cannot interconvert. =>
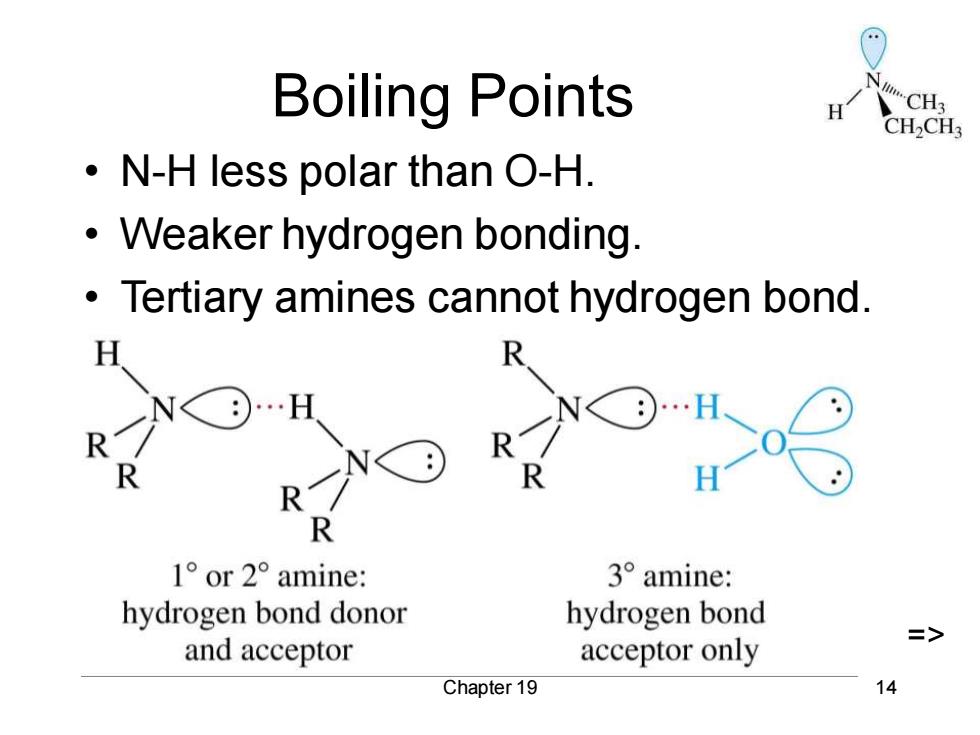
Boiling Points H CH2CH3 N-H less polar than O-H. Weaker hydrogen bonding. Tertiary amines cannot hydrogen bond. R H R 1°or2°amine: 3°amine: hydrogen bond donor hydrogen bond and acceptor acceptor only Chapter 19 14
Chapter 19 14 Boiling Points • N-H less polar than O-H. • Weaker hydrogen bonding. • Tertiary amines cannot hydrogen bond. =>

H CH3 Solubility and Odor CH2CH3 Small amines (<6 C)soluble in water. All amines accept hydrogen bonds from water and alcohol. Branching increases solubility. Most amines smell like rotting fish. NHCHCHCHCHCHNH2 1,5-pentanediamine or cadaverine Chapter 19 15
Chapter 19 15 Solubility and Odor • Small amines (<6 C) soluble in water. • All amines accept hydrogen bonds from water and alcohol. • Branching increases solubility. • Most amines smell like rotting fish. NH2 CH2 CH2 CH2 CH2 CH2 NH2 1,5-pentanediamine or cadaverine =>