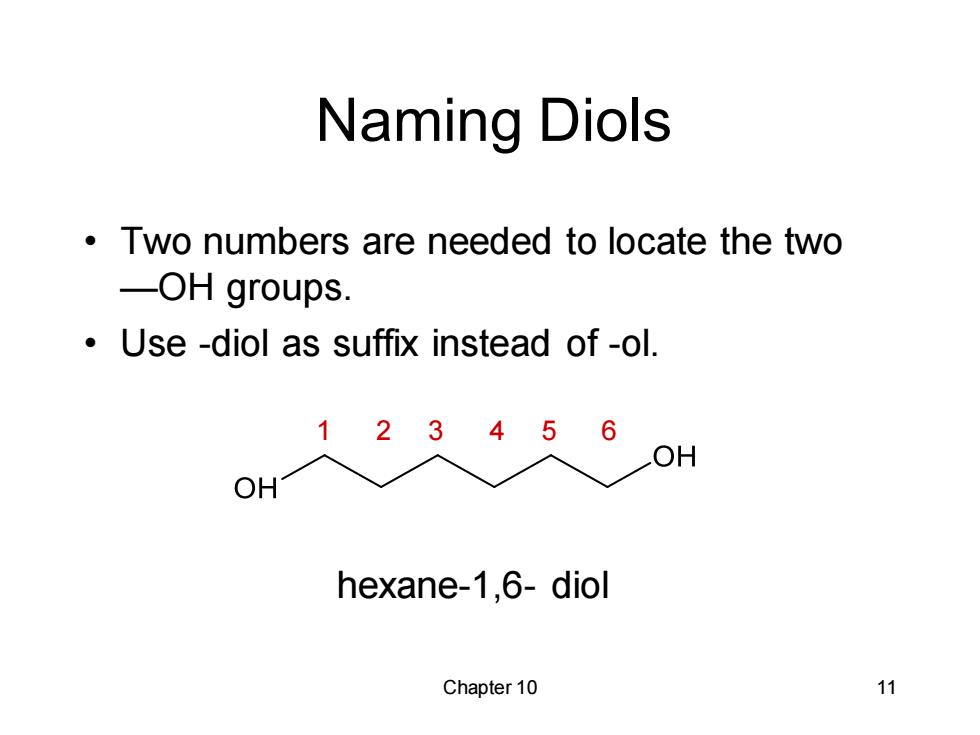
Naming Diols Two numbers are needed to locate the two -OH groups. Use -diol as suffix instead of -ol 1 2345 6 OH OH hexane-1,6-diol Chapter 10 11
Chapter 10 11 Naming Diols • Two numbers are needed to locate the two —OH groups. • Use -diol as suffix instead of -ol. hexane-1,6- diol 1 2 3 4 5 6
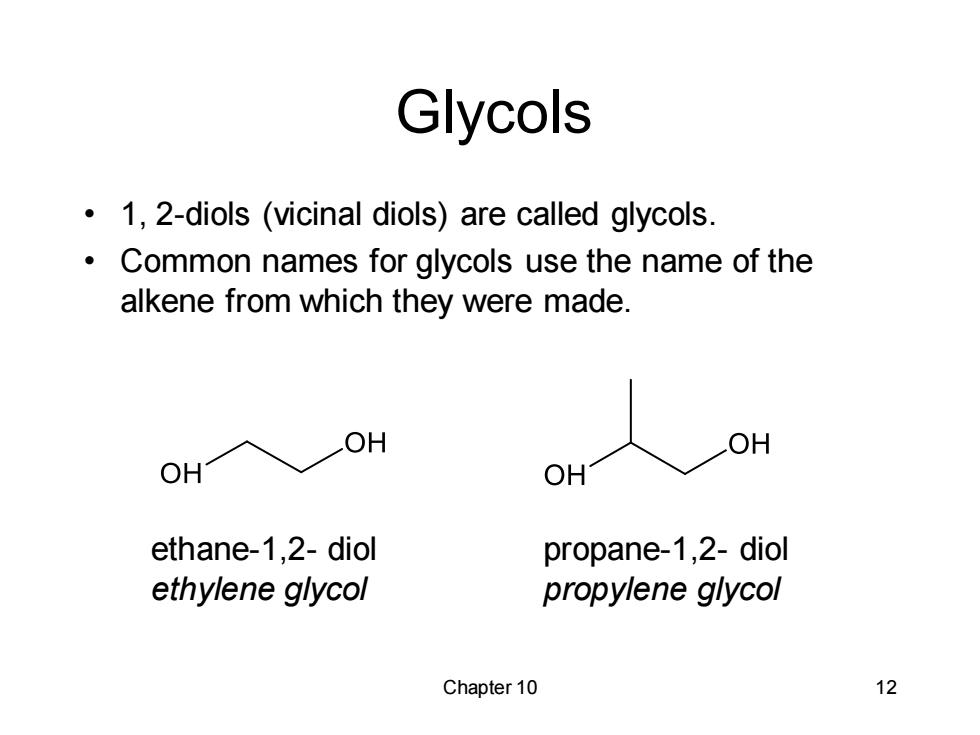
Glycols 1,2-diols (vicinal diols)are called glycols. Common names for glycols use the name of the alkene from which they were made. OH OH OH ethane-1,2-diol propane-1,2-diol ethylene glycol propylene glycol Chapter 10 12
Chapter 10 12 Glycols • 1, 2-diols (vicinal diols) are called glycols. • Common names for glycols use the name of the alkene from which they were made. ethane-1,2- diol ethylene glycol propane-1,2- diol propylene glycol

Phenol Nomenclature .-OH group is assumed to be on carbon 1. For common names of disubstituted phenols, use ortho-for 1,2;meta-for 1,3;and para-for 1,4 Methyl phenols are cresols. OH OH H3C CI 3-chlorophenol 4-methylphenol (meta-chlorophenol) (para-cresol)) Chapter 10 13
Chapter 10 13 Phenol Nomenclature • —OH group is assumed to be on carbon 1. • For common names of disubstituted phenols, use ortho- for 1,2; meta- for 1,3; and para- for 1,4. • Methyl phenols are cresols. 3-chlorophenol (meta-chlorophenol) 4-methylphenol (para-cresol) OH Cl OH H3C

Solved Problem 1 Give the systematic (IUPAC)name for the following alcohol. CHI CH,-OH CH,一CH2一CH—CH-CH一CH CH Solution The longest chain contains six carbon atoms,but it does not contain the carbon bonded to the hydroxyl group.The longest chain containing the carbon bonded to the-OH group is the one outlined by the green box,containing five carbon atoms.This chain is numbered from right to left in order to give the hydroxyl-bearing carbon atom the lowest possible number. OH 5CH3-CH2-3CH-2CH- CH-CH; CH The correct name for this compound is 3-(iodomethyl)-2-isopropylpentan-1-ol. Chapter 10 14
Chapter 10 14 Give the systematic (IUPAC) name for the following alcohol. The longest chain contains six carbon atoms, but it does not contain the carbon bonded to the hydroxyl group. The longest chain containing the carbon bonded to the —OH group is the one outlined by the green box, containing five carbon atoms. This chain is numbered from right to left in order to give the hydroxyl-bearing carbon atom the lowest possible number. The correct name for this compound is 3-(iodomethyl)-2-isopropylpentan-1-ol. Solved Problem 1 Solution

Physical Properties Alcohols have high boiling points due to hydrogen bonding between molecules. Small alcohols are miscible in water,but solubility decreases as the size of the alkyl group increases. Chapter 10 15
Chapter 10 15 Physical Properties • Alcohols have high boiling points due to hydrogen bonding between molecules. • Small alcohols are miscible in water, but solubility decreases as the size of the alkyl group increases