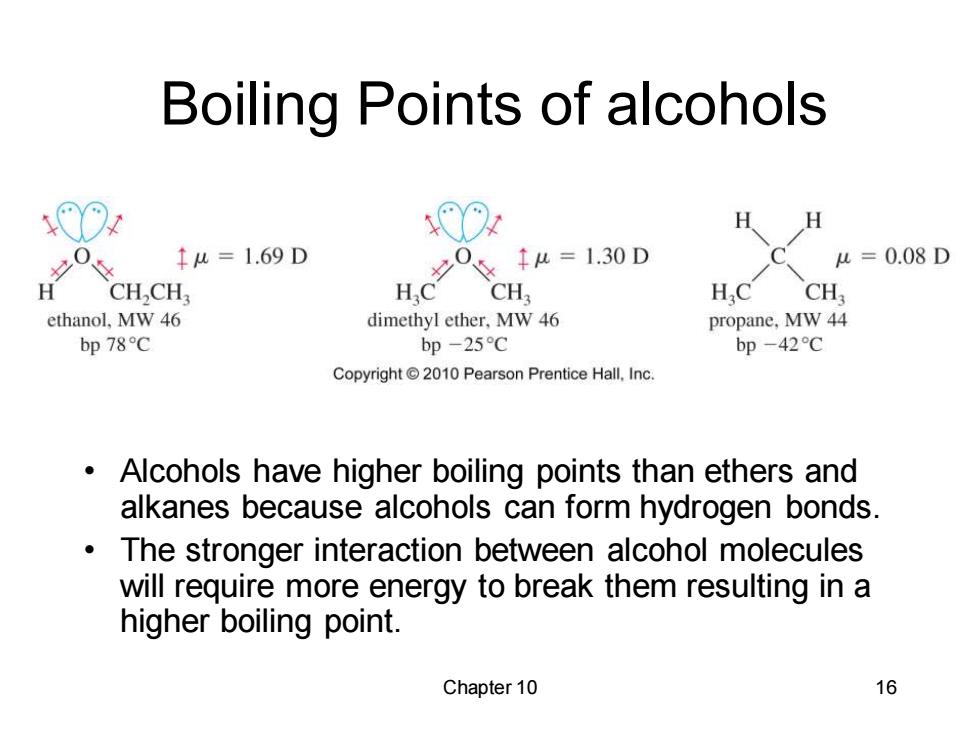
Boiling Points of alcohols H 0 1u=1.69D 1u=130D u=0.08D H CH,CH HC CH HC CH ethanol,MW 46 dimethyl ether,MW 46 propane,MW 44 bp78℃ bp-25C bp-42℃ Copyright2010 Pearson Prentice Hall.Inc. Alcohols have higher boiling points than ethers and alkanes because alcohols can form hydrogen bonds. The stronger interaction between alcohol molecules will require more energy to break them resulting in a higher boiling point. Chapter 10 16
Chapter 10 16 Boiling Points of alcohols • Alcohols have higher boiling points than ethers and alkanes because alcohols can form hydrogen bonds. • The stronger interaction between alcohol molecules will require more energy to break them resulting in a higher boiling point
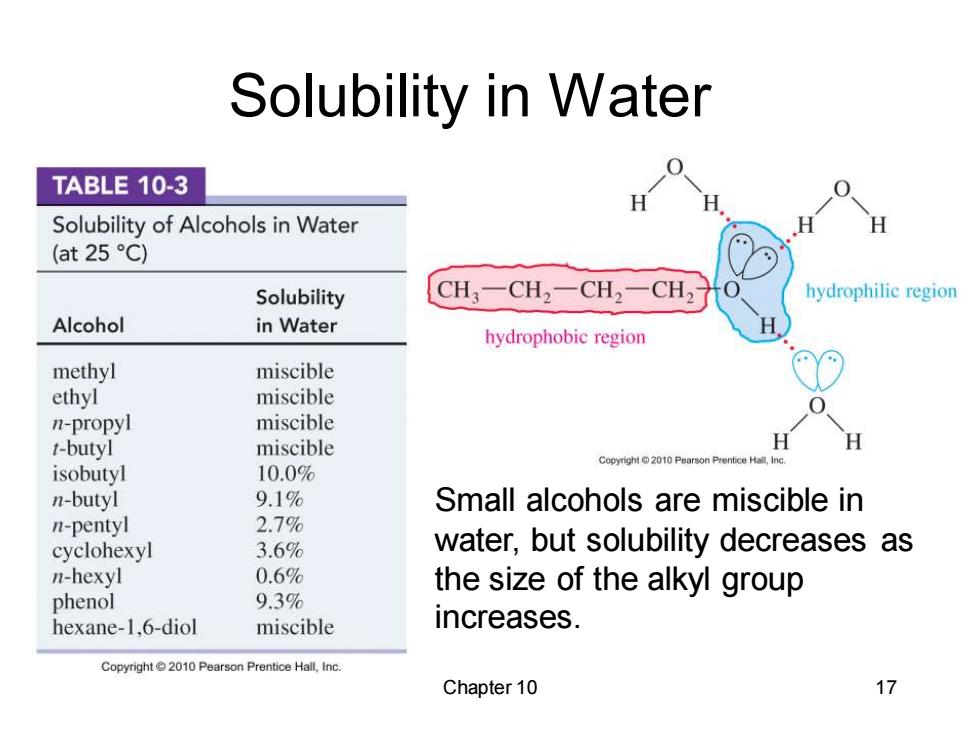
Solubility in Water TABLE 10-3 Solubility of Alcohols in Water H (at 25C) Solubility CH3一CH2一CH2一CH hydrophilic region Alcohol in Water hydrophobic region methyl miscible ethyl miscible n-propyl miscible t-butyl miscible H H Copyright2010 Pearson Prentice Hall,Inc isobutyl 10.0% n-butyl 9.1% Small alcohols are miscible in n-pentyl 2.7% cyclohexyl 3.6% water,but solubility decreases as n-hexyl 0.6% the size of the alkyl group phenol 9.3% hexane-1,6-diol miscible increases. Copyright2010 Pearson Prentice Hall,Inc. Chapter 10 17
Chapter 10 17 Solubility in Water Small alcohols are miscible in water, but solubility decreases as the size of the alkyl group increases

Methanol ·Vood alcohol" Industrial production from synthesis gas Common industrial solvent Toxic Dose:100 mL methanol Used as fuel at Indianapolis 500 Fire can be extinguished with water High octane rating ·Low emissions Lower energy content Invisible flame Chapter 10 18
Chapter 10 18 Methanol • “Wood alcohol” • Industrial production from synthesis gas • Common industrial solvent • Toxic Dose: 100 mL methanol • Used as fuel at Indianapolis 500 ▪ Fire can be extinguished with water ▪ High octane rating ▪ Low emissions ▪ Lower energy content ▪ Invisible flame