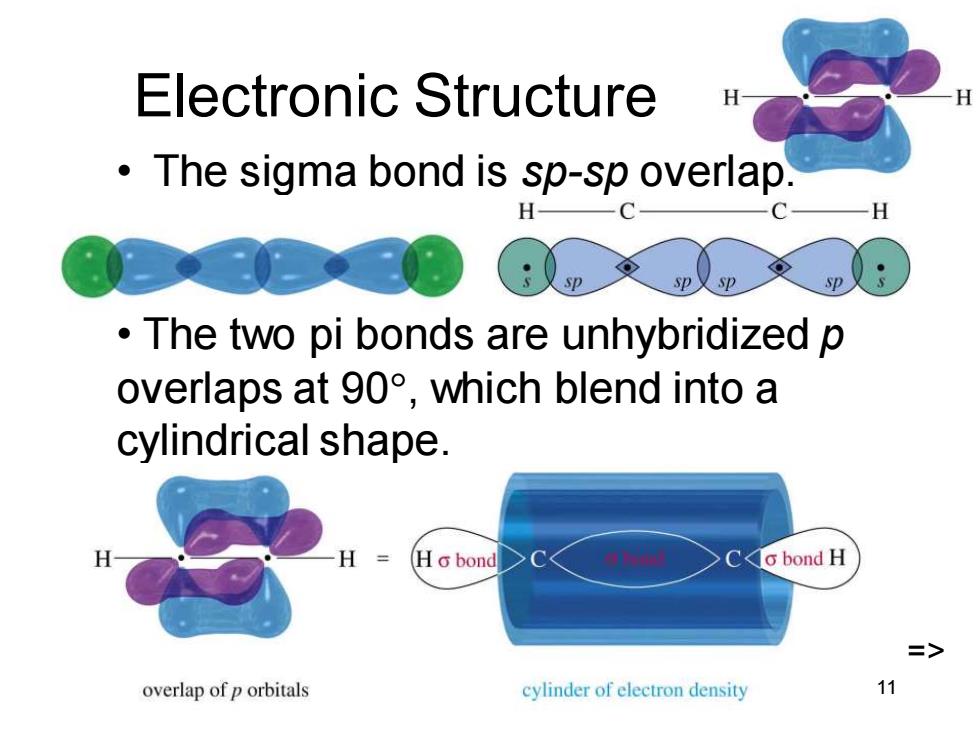
Electronic Structure The sigma bond is sp-sp overlap. C 一H p The two pi bonds are unhybridized p overlaps at90°,which blend into a cylindrical shape. HG bond Co bond H => overlap of p orbitals cylinder of electron density 11
Chapter 9 11 Electronic Structure • The sigma bond is sp-sp overlap. • The two pi bonds are unhybridized p overlaps at 90, which blend into a cylindrical shape. =>

H Bond Lengths More s character,so shorter length. Three bonding overlaps,so shorter. -1.54A 1.33A -1.20A H H H H H一C兰C一H H H 1.09A 1.08A 1.06 ethane ethene ethyne Bond angle is180°,so linear geometry. => Chapter 9 12
Chapter 9 12 Bond Lengths • More s character, so shorter length. • Three bonding overlaps, so shorter. Bond angle is 180, so linear geometry. =>

Acidity of Alkynes Terminal alkynes,R-C=C-H,are more acidic than other hydrocarbons. ·Acetylene-→acetylide by NH2,but not by OH-or RO. More s character,so pair of electrons in anion is held more closely to the nucleus.Less charge separation,so more stable. => Chapter 9 13
Chapter 9 13 Acidity of Alkynes • Terminal alkynes, R-CC-H, are more acidic than other hydrocarbons. • Acetylene → acetylide by NH2 - , but not by OH- or RO- . • More s character, so pair of electrons in anion is held more closely to the nucleus. Less charge separation, so more stable. =>
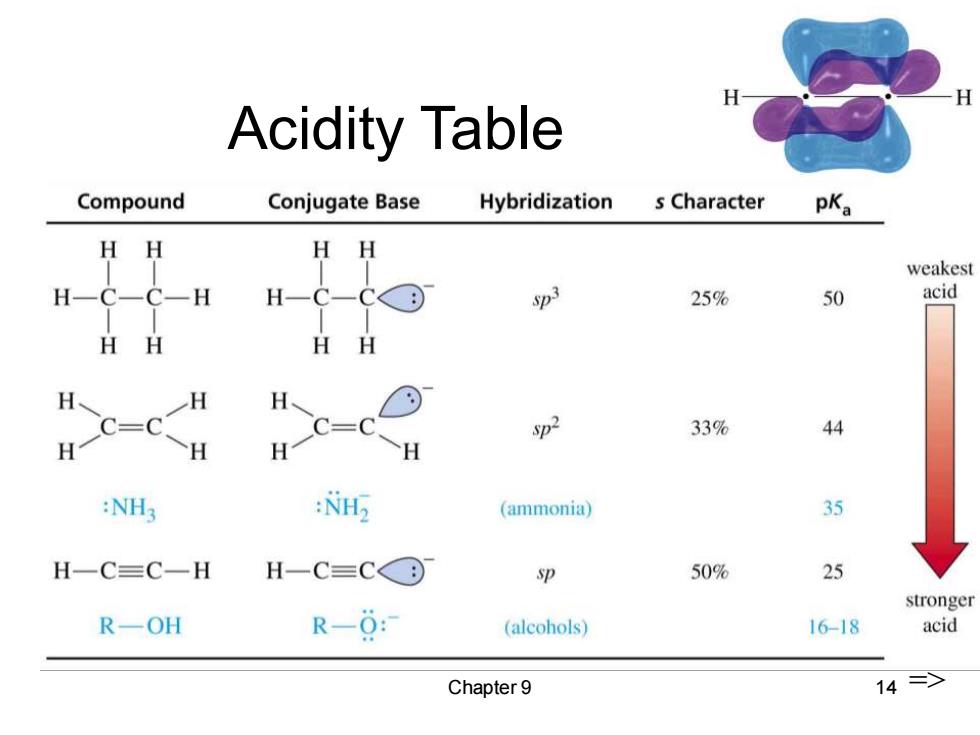
H Acidity Table Compound Conjugate Base Hybridization s Character pKa HH weakest H-C-C-H H-C-CC© Sp3 25% 50 acid HH HH c-c H H-c=c H 2 33% 44 :NH3 :NH2 (ammonia】 35 H一C=C一H H一C=CC⊙ p 50% 25 stronger R-OH R-0: (alcohols) 16-18 acid Chapter 9 14=>
Chapter 9 14 Acidity Table =>

Forming Acetylide lons H+can be removed from a terminal alkyne by sodium amide,NaNH2. CH3-C=C-H NaNH2 CH3-C=C:Na*NH3 NaNH,is produced by the reaction of ammonia with sodium metal. H H H--H+M的 53 H-☑本+NH时 => Chapter 9 15
Chapter 9 15 Forming Acetylide Ions • H+ can be removed from a terminal alkyne by sodium amide, NaNH2 . CH3 C C H + NaNH2 CH3 C C: - Na + + NH3 • NaNH2 is produced by the reaction of ammonia with sodium metal. N H H H + Na Fe +3 N + 1/ 2 H2 H H Na + =>