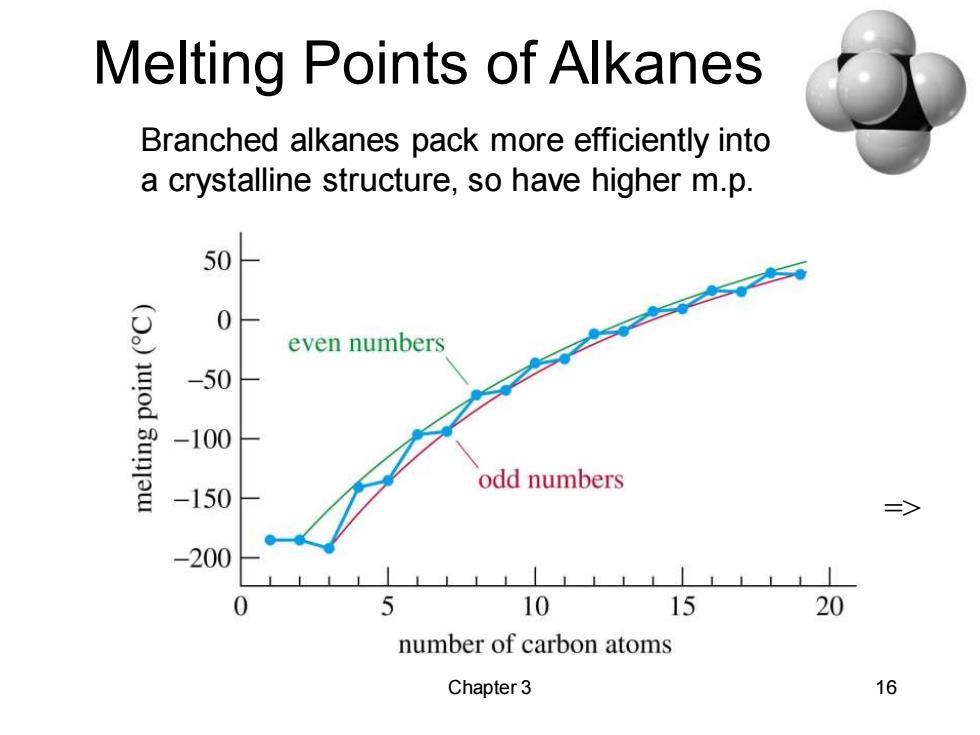
Melting Points of Alkanes Branched alkanes pack more efficiently into a crystalline structure,so have higher m.p. 50 0 even numbers uod -50 Sunjew -100 odd numbers -150 => -200 5 10 15 20 number of carbon atoms Chapter 3 16
Chapter 3 16 Melting Points of Alkanes Branched alkanes pack more efficiently into a crystalline structure, so have higher m.p. =>
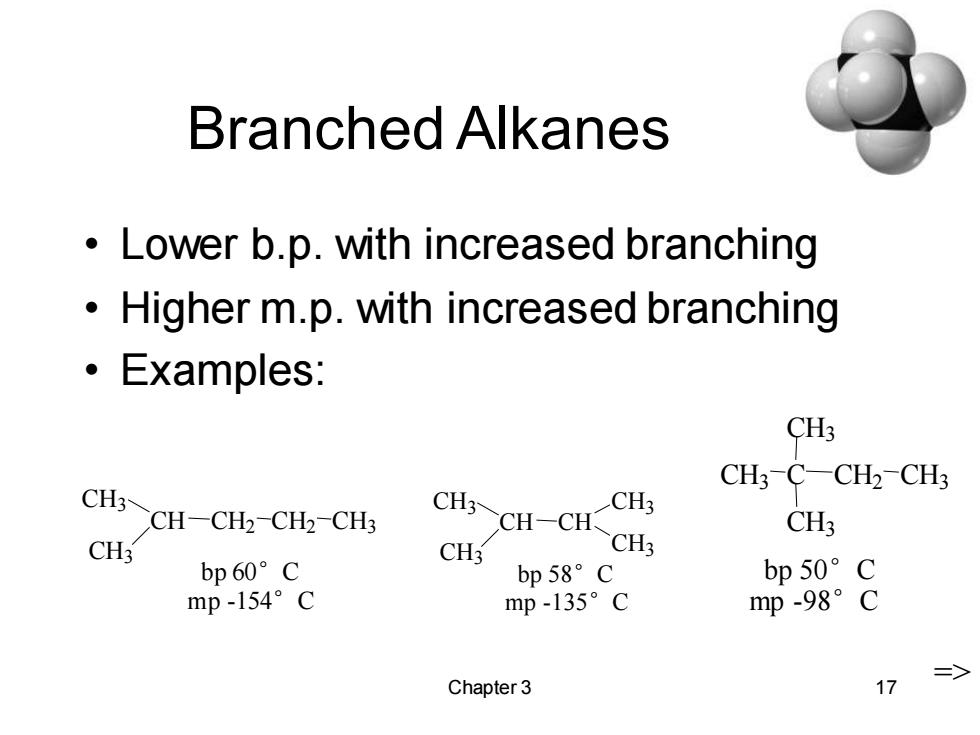
Branched Alkanes Lower b.p.with increased branching Higher m.p.with increased branching ·Examples: CH3 CH3-C-CH2-CH3 CH3~CH-CH2-CH2-CHs CHCH一CH CH3 CH3 CH3 CH3 CH3 bp60°C bp58°c bp50°c mp-154°C mp-135°c mp-98°C => Chapter 3 17
Chapter 3 17 Branched Alkanes • Lower b.p. with increased branching • Higher m.p. with increased branching • Examples: H CH3 CH CH3 CH2 CH2 CH3 bp 60°C mp -154°C CH3 CH CH3 CH CH3 CH3 bp 58°C mp -135°C => bp 50°C mp -98°C CH3 C C 3 CH3 CH2 CH3
Major Uses of Alkanes C-C2:gases (natural gas) C3-C4:liquified petroleum(LPG) C5-Ce:gasoline C-C16:diesel,kerosene,jet fuel C17-up:lubricating oils,heating oil Origin:petroleum refining => Chapter 3 18
Chapter 3 18 Major Uses of Alkanes • C1 -C2 : gases (natural gas) • C3 -C4 : liquified petroleum (LPG) • C5 -C8 : gasoline • C9 -C16: diesel, kerosene, jet fuel • C17-up: lubricating oils, heating oil • Origin: petroleum refining =>