Depicting Carbohydrate Stereochemistry: Fischer Projections Three steps for assigning R,S stereochemical designations in Fischer projections Step 1 Assign priorities to the four substituents in the usual way Step 2 Place the group of lowest priority,usually H,at the top of the Fischer projection by using one of the allowed motions The lowest-priority group is thus oriented back away from viewer Step 3 Determine the direction of rotation 1-2-3 of the remaining three groups and assign R or S configuration
Three steps for assigning R,S stereochemical designations in Fischer projections Step 1 Assign priorities to the four substituents in the usual way Step 2 Place the group of lowest priority, usually H, at the top of the Fischer projection by using one of the allowed motions ▪ The lowest-priority group is thus oriented back away from viewer Step 3 Determine the direction of rotation 1→2→3 of the remaining three groups and assign R or S configuration Depicting Carbohydrate Stereochemistry: Fischer Projections
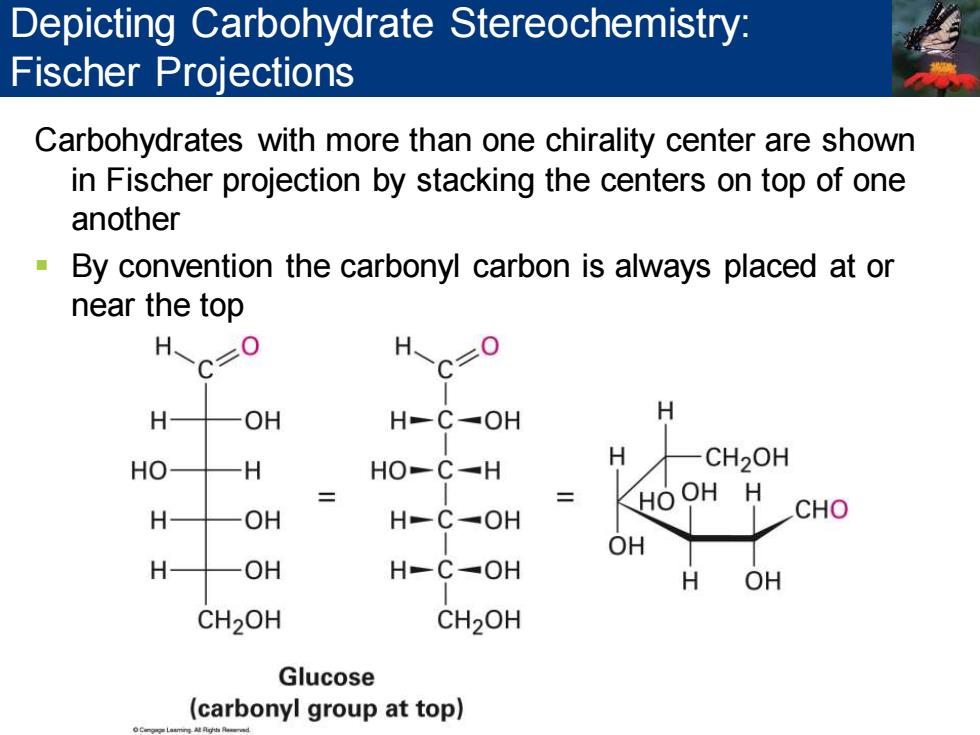
Depicting Carbohydrate Stereochemistry: Fischer Projections Carbohydrates with more than one chirality center are shown in Fischer projection by stacking the centers on top of one another By convention the carbonyl carbon is always placed at or near the top H OH H-C✉OH H HO H HO-C-H H CH2OH HOOH H H OH H-C-OH CHO OH H OH HC✉OH H OH CH2OH CH2OH Glucose (carbonyl group at top)
Carbohydrates with more than one chirality center are shown in Fischer projection by stacking the centers on top of one another ▪ By convention the carbonyl carbon is always placed at or near the top Depicting Carbohydrate Stereochemistry: Fischer Projections
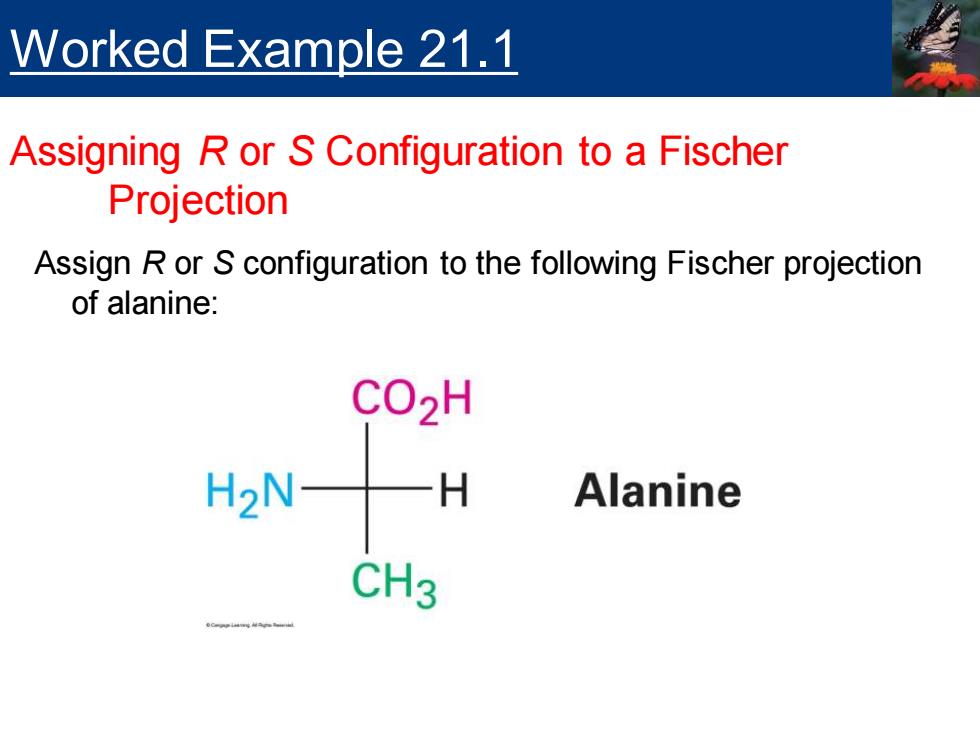
Worked Example 21.1 Assigning R or S Configuration to a Fischer Projection Assign R or S configuration to the following Fischer projection of alanine: CO2H H2NH Alanine CH3
Assign R or S configuration to the following Fischer projection of alanine: Worked Example 21.1 Assigning R or S Configuration to a Fischer Projection
Worked Example 21.1 Assigning R or S Configuration to a Fischer Projection Strategy Follow the steps listed in the text 1.Assign priorities to the four substituents on the chiral carbon 2.Manipulate the Fischer projection to place the group of lowest priority at the top by carrying out one of the allowed motions 3.Determine the direction 1-23 of the remaining three groups
Strategy ▪ Follow the steps listed in the text 1. Assign priorities to the four substituents on the chiral carbon 2. Manipulate the Fischer projection to place the group of lowest priority at the top by carrying out one of the allowed motions 3. Determine the direction 1→2→3 of the remaining three groups Worked Example 21.1 Assigning R or S Configuration to a Fischer Projection
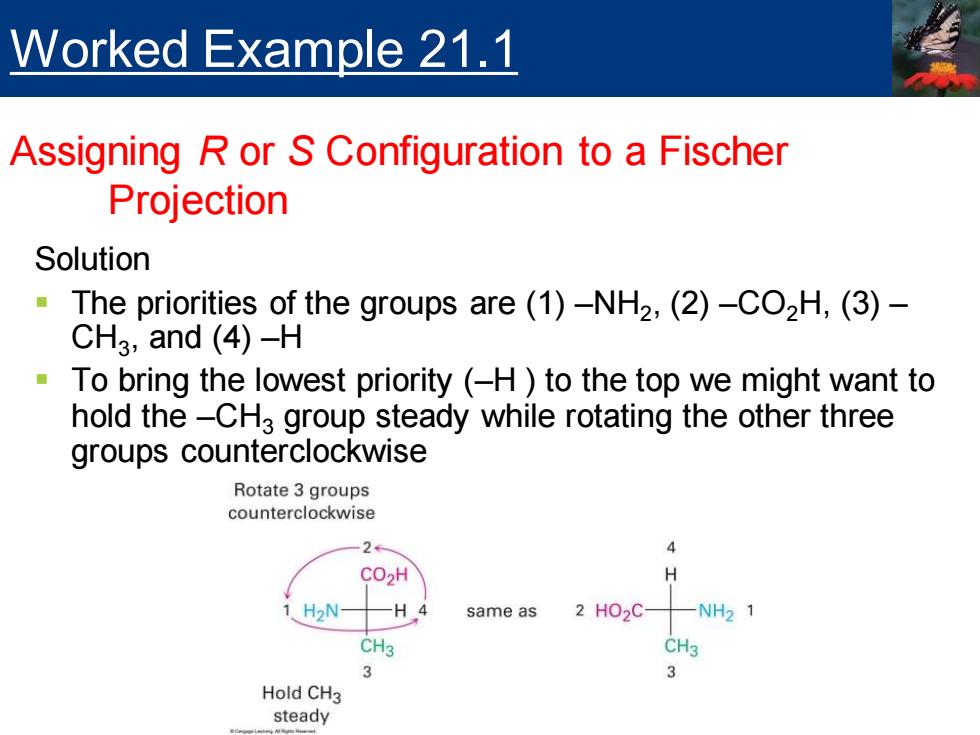
Worked Example 21.1 Assigning R or S Configuration to a Fischer Projection Solution The priorities of the groups are(1)-NH2,(2)-CO2H,(3)- CH3,and (4)-H To bring the lowest priority(-H to the top we might want to hold the-CH3 group steady while rotating the other three groups counterclockwise Rotate 3 groups counterclockwise 2 4 CO2H H 1 H2N- -H4 same as 2H02C -NH21 CH3 CH3 3 Hold CH3 steady
Solution ▪ The priorities of the groups are (1) –NH2 , (2) –CO2H, (3) – CH3 , and (4) –H ▪ To bring the lowest priority (–H ) to the top we might want to hold the –CH3 group steady while rotating the other three groups counterclockwise Worked Example 21.1 Assigning R or S Configuration to a Fischer Projection