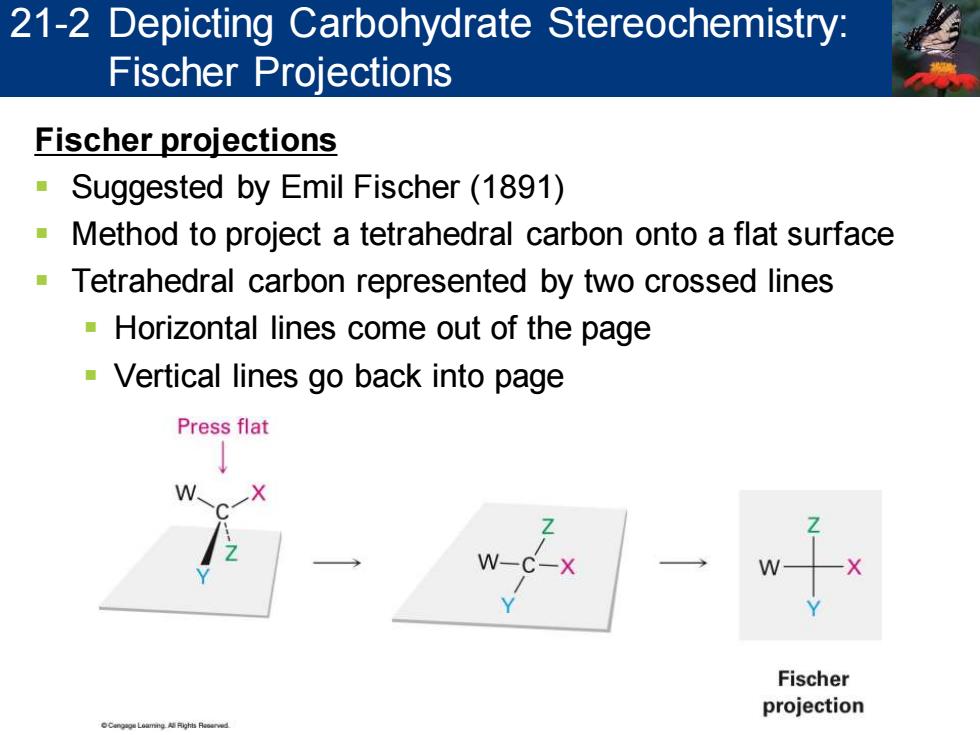
21-2 Depicting Carbohydrate Stereochemistry: Fischer Projections Fischer projections Suggested by Emil Fischer(1891) Method to project a tetrahedral carbon onto a flat surface Tetrahedral carbon represented by two crossed lines Horizontal lines come out of the page -Vertical lines go back into page Press flat Fischer projection
Fischer projections ▪ Suggested by Emil Fischer (1891) ▪ Method to project a tetrahedral carbon onto a flat surface ▪ Tetrahedral carbon represented by two crossed lines ▪ Horizontal lines come out of the page ▪ Vertical lines go back into page 21-2 Depicting Carbohydrate Stereochemistry: Fischer Projections
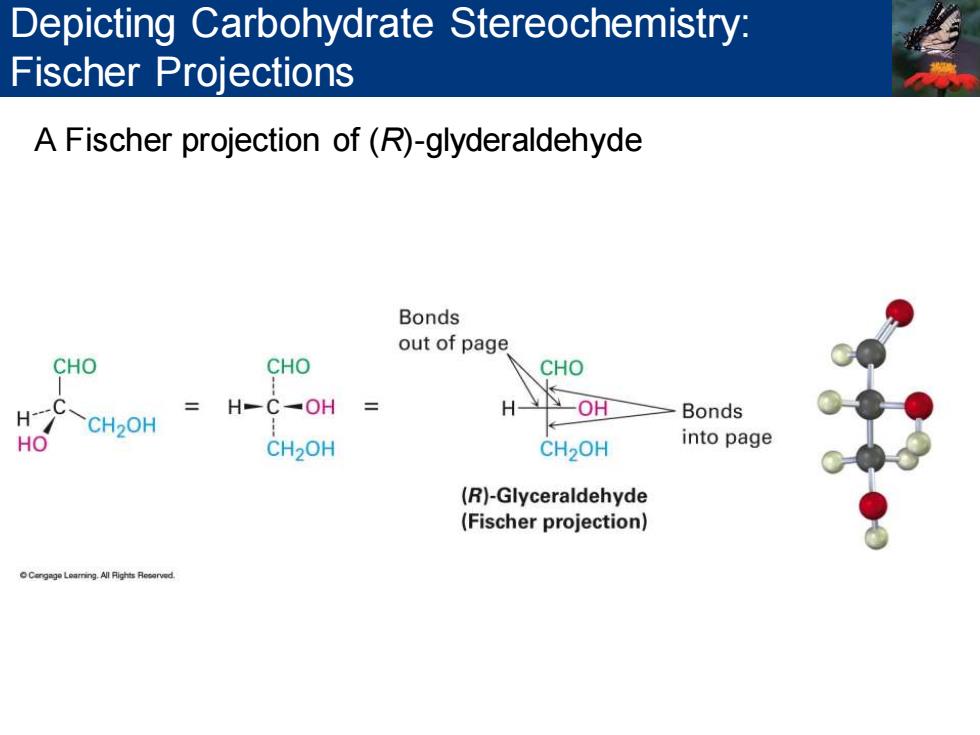
Depicting Carbohydrate Stereochemistry: Fischer Projections A Fischer projection of(R)-glyderaldehyde Bonds out of page CHO CHO CHO H H-C-OH H -OH Bonds CH2OH HO CH2OH CH>OH into page (R)-Glyceraldehyde (Fischer projection) Cengag Learring.All Fights Reerved
A Fischer projection of (R)-glyderaldehyde Depicting Carbohydrate Stereochemistry: Fischer Projections
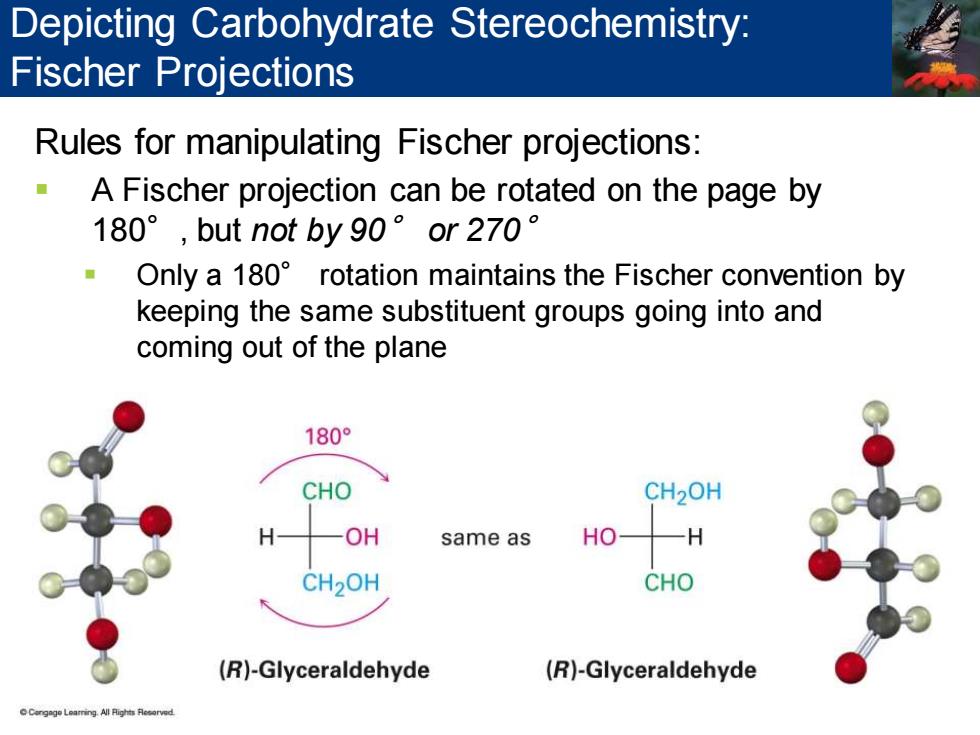
Depicting Carbohydrate Stereochemistry: Fischer Projections Rules for manipulating Fischer projections: A Fischer projection can be rotated on the page by 180°,but not by90°or270° Only a 180 rotation maintains the Fischer convention by keeping the same substituent groups going into and coming out of the plane 180° CHO CH2OH H OH same as HO -H CH2OH CHO (R)-Glyceraldehyde (R)-Glyceraldehyde
Rules for manipulating Fischer projections: ▪ A Fischer projection can be rotated on the page by 180°, but not by 90° or 270° ▪ Only a 180° rotation maintains the Fischer convention by keeping the same substituent groups going into and coming out of the plane Depicting Carbohydrate Stereochemistry: Fischer Projections
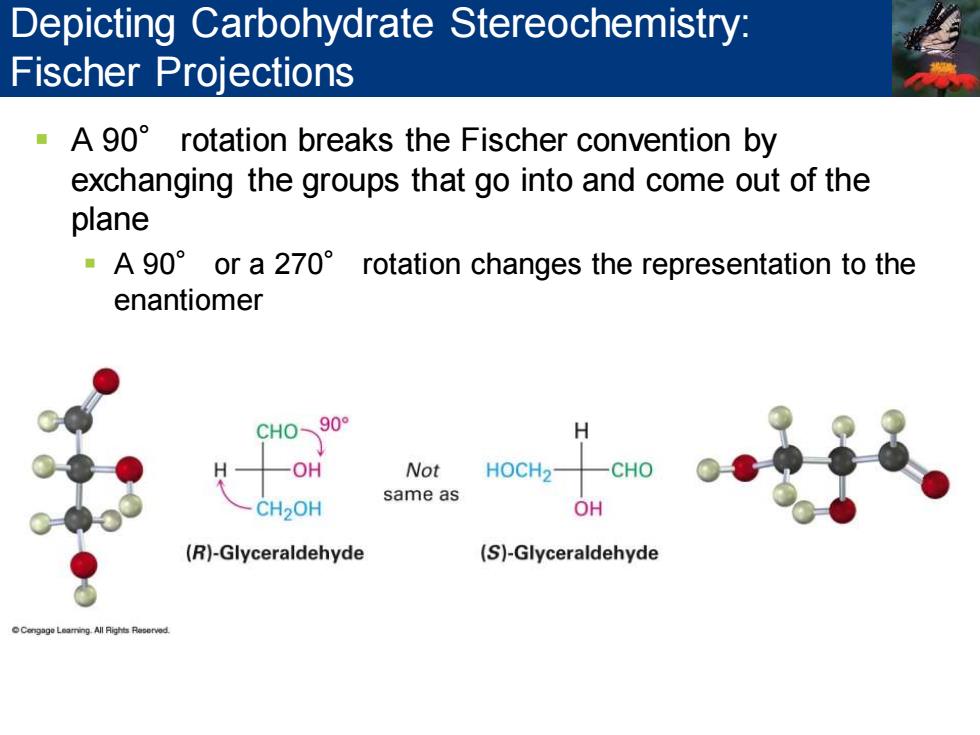
Depicting Carbohydrate Stereochemistry: Fischer Projections A 90 rotation breaks the Fischer convention by exchanging the groups that go into and come out of the plane A 90 or a 270 rotation changes the representation to the enantiomer CH090 H OH Not HOCH2- -CHO same as CH2OH OH (R)-Glyceraldehyde (S)-Glyceraldehyde
▪ A 90° rotation breaks the Fischer convention by exchanging the groups that go into and come out of the plane ▪ A 90° or a 270° rotation changes the representation to the enantiomer Depicting Carbohydrate Stereochemistry: Fischer Projections
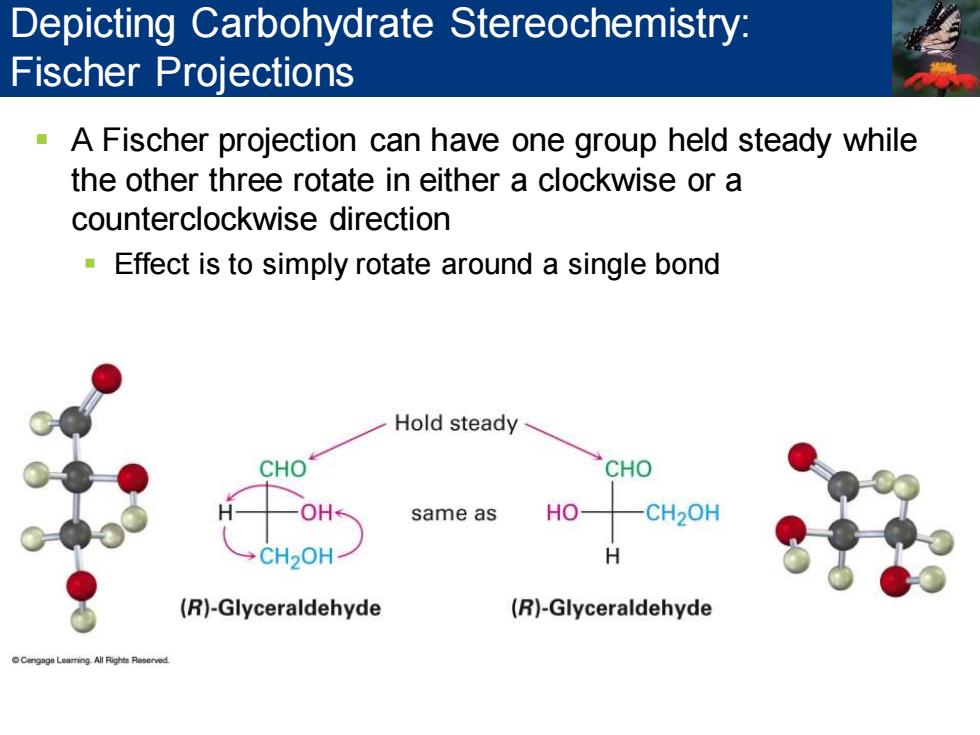
Depicting Carbohydrate Stereochemistry: Fischer Projections A Fischer projection can have one group held steady while the other three rotate in either a clockwise or a counterclockwise direction Effect is to simply rotate around a single bond Hold steady CHO CHO OH same as HO CH2OH →CH20H H (R)-Glyceraldehyde (R)-Glyceraldehyde
▪ A Fischer projection can have one group held steady while the other three rotate in either a clockwise or a counterclockwise direction ▪ Effect is to simply rotate around a single bond Depicting Carbohydrate Stereochemistry: Fischer Projections