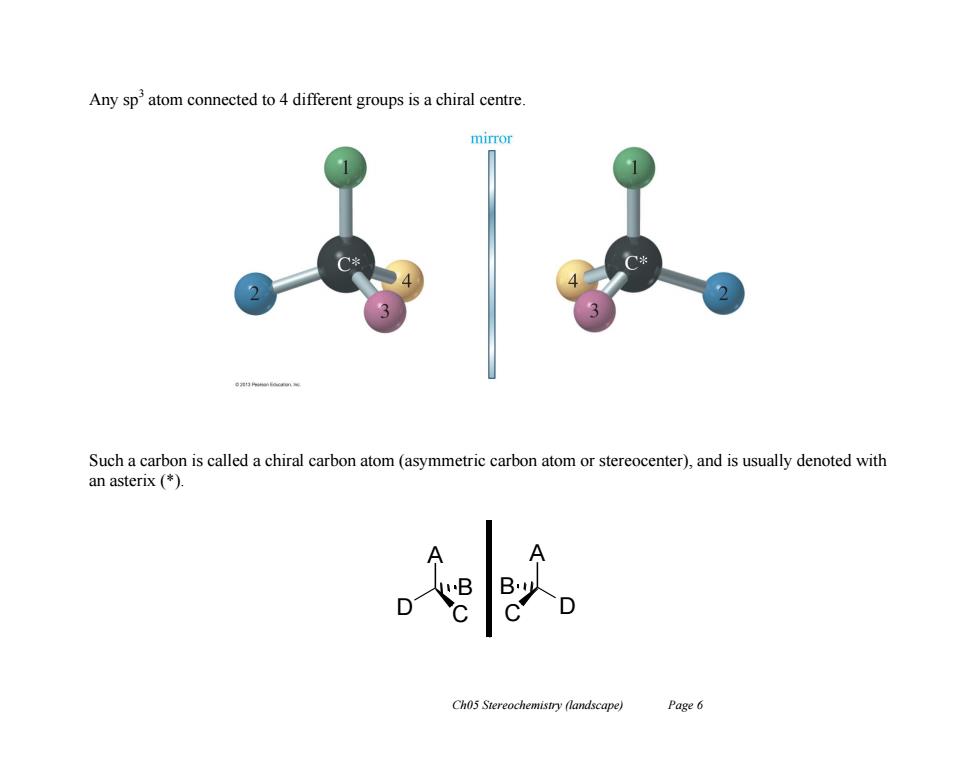
Any sp2 atom connected to 4 different groups is a chiral centre. mirror Such a carbon is called a chiral carbon atom(asymmetric carbon atom or stereocenter),and is usually denoted with an asterix (*) Ch05 Stereochemistry (landscape) Page 6
Ch05 Stereochemistry (landscape) Page 6 Any sp3 atom connected to 4 different groups is a chiral centre. Such a carbon is called a chiral carbon atom (asymmetric carbon atom or stereocenter), and is usually denoted with an asterix (*). A C D B A D C B

(R)and (S)Assignments To distinguish between the two enantiomers,one is called the(S)enantiomer,and the other the(R)enantiomer The(R)and(S)assignments are designated via the Cahn-Ingold-Prelog Convention. Each chiral center is designated either(R)or(S) There are two steps to assigning(R)or(S)to an enantiomer. 1)Assignment of"priority"to the groups bound to the asymmetric carbon 2)Using the“priority'”to decide on(R)or(S). 1)Assignment of Priority We look at the atoms directly bound to chiral carbon (a)Atoms with higher atomic numbers receive higher priorities. I>S>0>N>3℃>12C>Li>3H(T>2H(D)>H (b)In the case of the same atoms being bound directly to the chiral carbon,we go to the next atoms along the chain. -CH2Br>-CHCl2>-C(CH3)3>-CH(CH3)CH2F>-CH(CH3)2>-CH2CH; (c)Double and triple bonds are treated as if each bond were to a separate atom.(Imagine that each it bond is broken and that the atoms at both ends were duplicated). Ch05 Stereochemistry (landscape) Page 7
Ch05 Stereochemistry (landscape) Page 7 (R) and (S) Assignments To distinguish between the two enantiomers, one is called the (S) enantiomer, and the other the (R) enantiomer. The (R) and (S) assignments are designated via the Cahn-Ingold-Prelog Convention. Each chiral center is designated either (R) or (S). There are two steps to assigning (R) or (S) to an enantiomer: 1) Assignment of “priority” to the groups bound to the asymmetric carbon 2) Using the “priority” to decide on (R) or (S). 1) Assignment of Priority We look at the atoms directly bound to chiral carbon. (a) Atoms with higher atomic numbers receive higher priorities. I > S > O > N > 13C > 12C > Li > 3H (T) > 2H (D) > H (b) In the case of the same atoms being bound directly to the chiral carbon, we go to the next atoms along the chain. -CH2Br > -CHCl2 > -C(CH3)3 > -CH(CH3)CH2F > -CH(CH3)2 > -CH2CH3 (c) Double and triple bonds are treated as if each bond were to a separate atom. (Imagine that each bond is broken and that the atoms at both ends were duplicated)
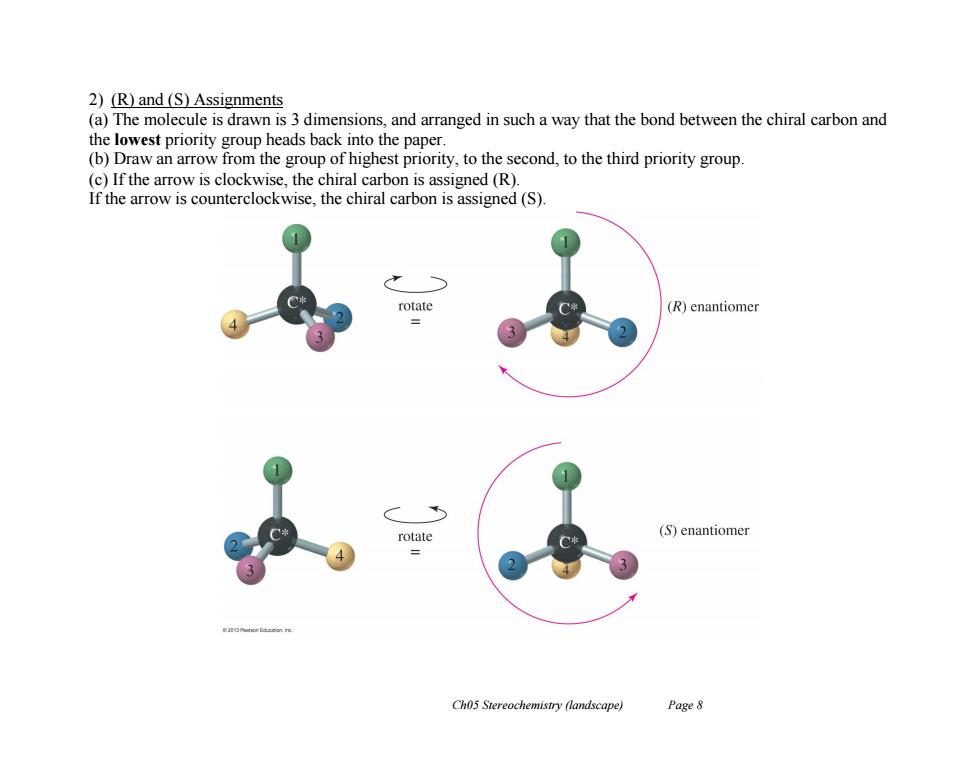
2)(R)and(S)Assignments (a)The molecule is drawn is 3 dimensions,and arranged in such a way that the bond between the chiral carbon and the lowest priority group heads back into the paper. (b)Draw an arrow from the group of highest priority,to the second,to the third priority group. (c)If the arrow is clockwise,the chiral carbon is assigned (R). If the arrow is counterclockwise,the chiral carbon is assigned (S) rotate (R)enantiomer rotate (S)enantiomer = Ch05 Stereochemistry (landscape) Page 8
Ch05 Stereochemistry (landscape) Page 8 2) (R) and (S) Assignments (a) The molecule is drawn is 3 dimensions, and arranged in such a way that the bond between the chiral carbon and the lowest priority group heads back into the paper. (b) Draw an arrow from the group of highest priority, to the second, to the third priority group. (c) If the arrow is clockwise, the chiral carbon is assigned (R). If the arrow is counterclockwise, the chiral carbon is assigned (S)
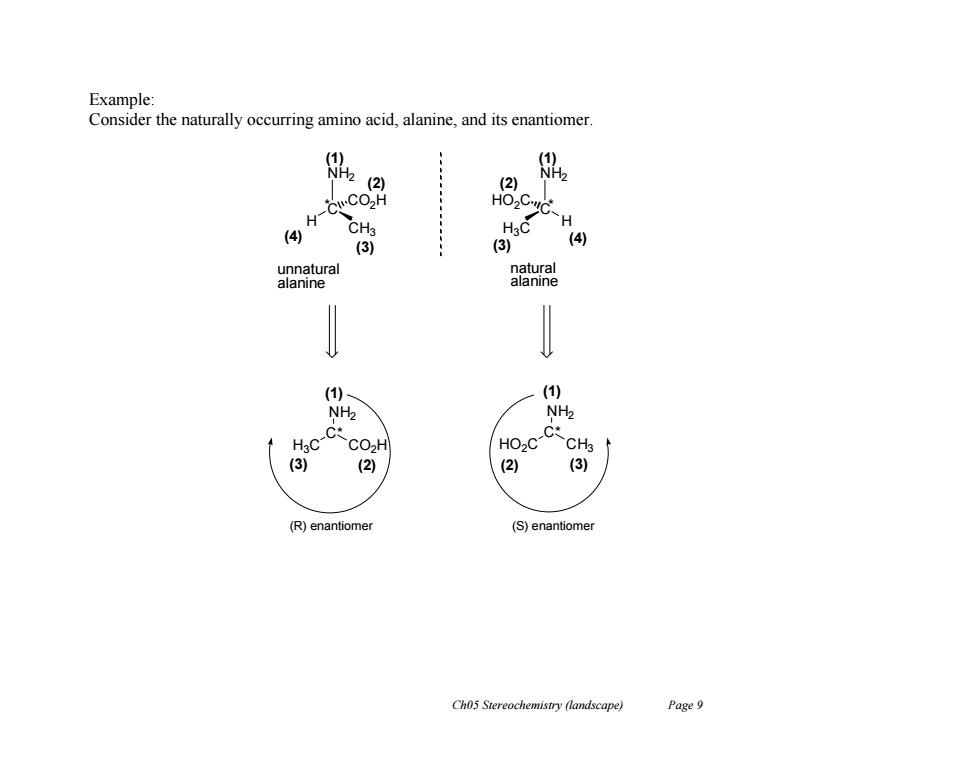
Example: Consider the naturally occurring amino acid,alanine,and its enantiomer (1 NH2 (2) 白 H CH3 H (4) (3) (3) (4) unnatural natural alanine alanine (1) (1) NH2 NH2 c-C CO2H HO2C-C CH3 (3) (2) ( (3) (R)enantiomer (S)enantiomer Ch05 Stereochemistry (landscape) Page 9
Ch05 Stereochemistry (landscape) Page 9 Example: Consider the naturally occurring amino acid, alanine, and its enantiomer. NH2 C H CO2H C* NH2 H3C CO2H * NH2 C H HO2C C* NH2 HO2C CH3 * (R) enantiomer (S) enantiomer CH3 H3C (1) (2) (3) (4) (1) (4) (2) (3) (1) (1) (3) (2) (2) (3) unnatural alanine natural alanine

Optical Activity Enantiomers have the same Boiling points Melting points Density. Almost all their physical properties are identical,but one exception is their effect on plane polarized light Enantiomeric compounds rotate the plane of polarized light in equal but opposite directions. Chiral compounds are called optically active compounds Optically active compounds which rotate plane polarized light clockwise (dextrorotatory)are designated (+)or d. Optically active compounds which rotate plane polarized light counterclockwise (levorotatory)are designated (- or I. Note:There is no relationship between(R)and(S)and the direction of the rotation of plane polarized light.(R) does not mean d and (+) (R)and(S)are just names from the CIP convention.(+)and(-)are experimentally observed physical properties. Ch05 Stereochemistry (landscape) Page 10
Ch05 Stereochemistry (landscape) Page 10 Optical Activity Enantiomers have the same Boiling points Melting points Density. Almost all their physical properties are identical, but one exception is their effect on plane polarized light. Enantiomeric compounds rotate the plane of polarized light in equal but opposite directions. Chiral compounds are called optically active compounds. Optically active compounds which rotate plane polarized light clockwise (dextrorotatory) are designated (+) or d. Optically active compounds which rotate plane polarized light counterclockwise (levorotatory) are designated (-) or l. Note: There is no relationship between (R) and (S) and the direction of the rotation of plane polarized light. (R) does not mean d and (+). (R) and (S) are just names from the CIP convention. (+) and (-) are experimentally observed physical properties