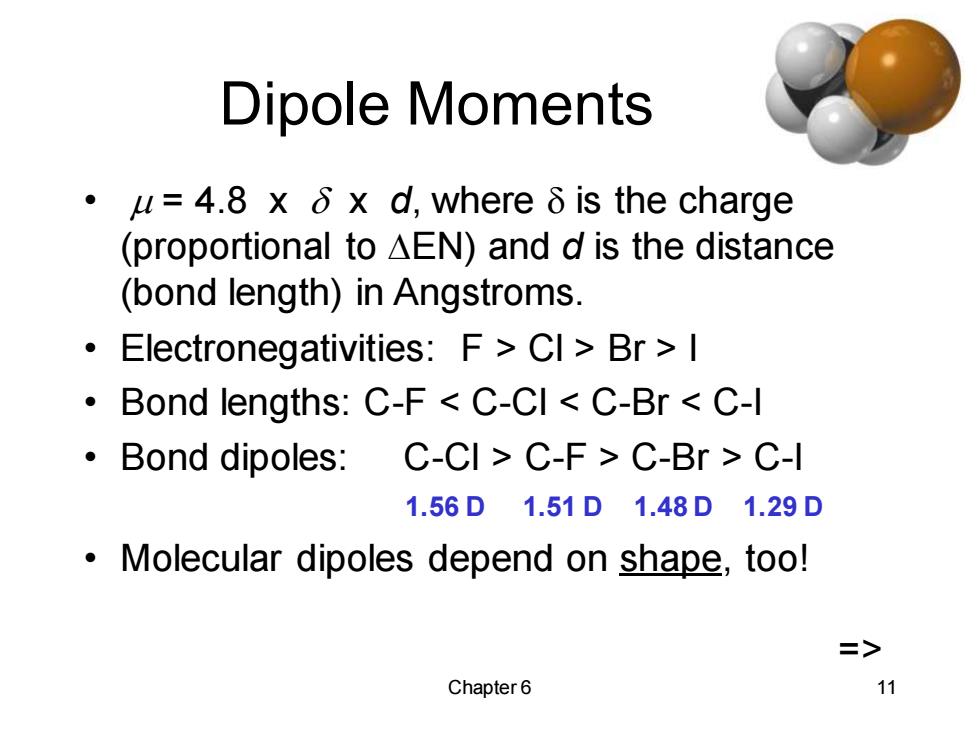
Dipole Moments ·u=4.8xδxd,whereδis the charge (proportional to AEN)and d is the distance (bond length)in Angstroms. Electronegativities:F>CI>Br >I Bond lengths:C-F C-CI C-Br C-I Bond dipoles:C-CI C-F C-Br C-I 1.56D1.51D1.48D1.29D ·Molecular dipoles depend on shape,too! => Chapter6 11
Chapter 6 11 Dipole Moments • m = 4.8 x x d, where is the charge (proportional to DEN) and d is the distance (bond length) in Angstroms. • Electronegativities: F > Cl > Br > I • Bond lengths: C-F < C-Cl < C-Br < C-I • Bond dipoles: C-Cl > C-F > C-Br > C-I 1.56 D 1.51 D 1.48 D 1.29 D • Molecular dipoles depend on shape, too! =>
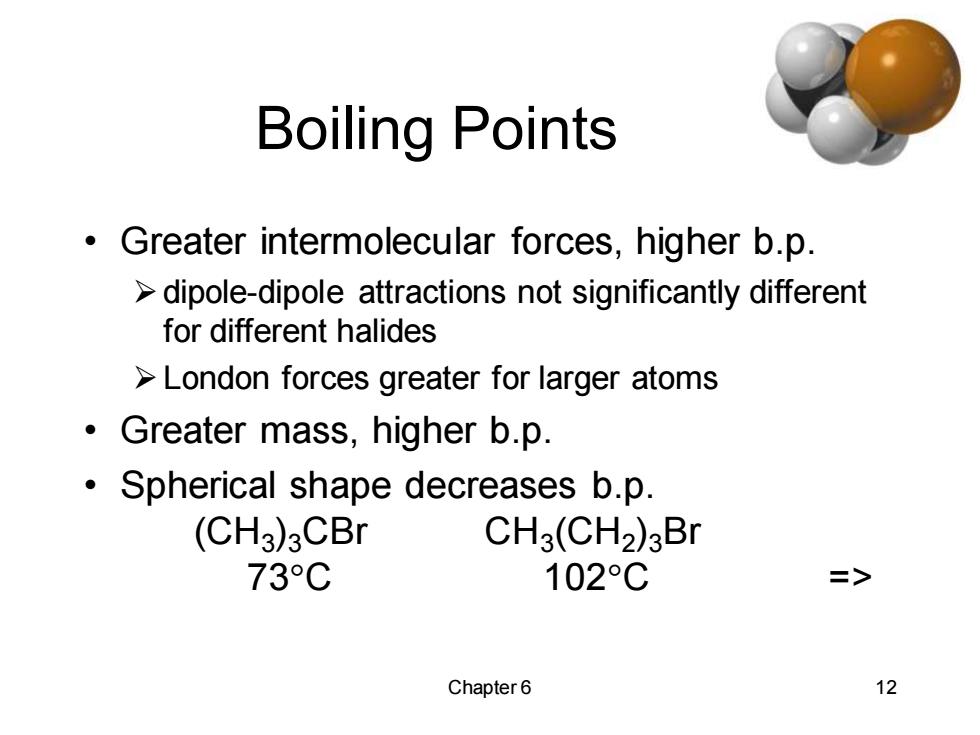
Boiling Points Greater intermolecular forces,higher b.p. dipole-dipole attractions not significantly different for different halides London forces greater for larger atoms Greater mass,higher b.p. 。 Spherical shape decreases b.p. (CH3)3CBr CH3(CH2)3Br 73°C 102°C => Chapter 6 12
Chapter 6 12 Boiling Points • Greater intermolecular forces, higher b.p. ➢dipole-dipole attractions not significantly different for different halides ➢London forces greater for larger atoms • Greater mass, higher b.p. • Spherical shape decreases b.p. (CH3 )3CBr CH3 (CH2 )3Br 73C 102C =>

Densities Alkyl fluorides and chlorides less dense than water. Alkyl dichlorides,bromides,and iodides more dense than water. => Chapter6 13
Chapter 6 13 Densities • Alkyl fluorides and chlorides less dense than water. • Alkyl dichlorides, bromides, and iodides more dense than water. =>

Preparation of RX Free radical halogenation(Chapter 4) >produces mixtures,not good lab synthesis >unless:all H's are equivalent,or >halogenation is highly selective. Free radical allylic halogenation >produces alkyl halide with double bond on the neighboring carbon. > Chapter6 14
Chapter 6 14 Preparation of RX • Free radical halogenation (Chapter 4) ➢produces mixtures, not good lab synthesis ➢unless: all H’s are equivalent, or ➢halogenation is highly selective. • Free radical allylic halogenation ➢produces alkyl halide with double bond on the neighboring carbon. =>
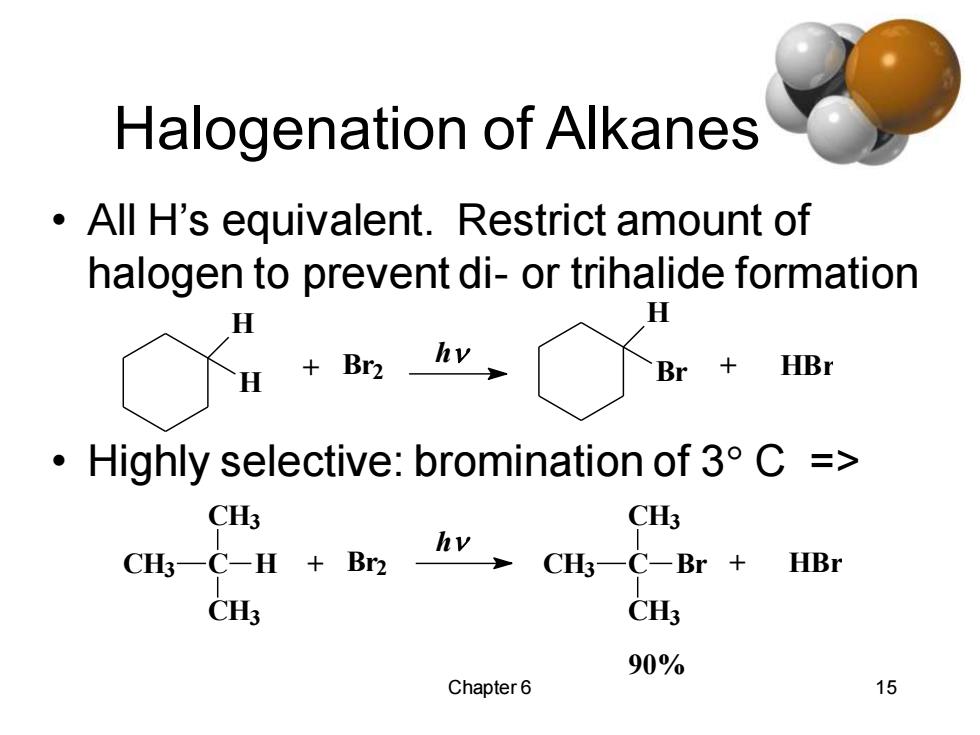
Halogenation of Alkanes All H's equivalent.Restrict amount of halogen to prevent di-or trihalide formation H H H +Br2 hv Br HBr ·Highly selective:bromination of3°C: => CH3 CH3 CH3一C-H+Br2 CH一C-Br+ HBr CH3 CH3 90% Chapter6 15
Chapter 6 15 Halogenation of Alkanes • All H’s equivalent. Restrict amount of halogen to prevent di- or trihalide formation • Highly selective: bromination of 3 C => + HBr H Br h + Br2 H H 90% CH3 C + HBr CH3 CH3 Br h CH3 C + Br2 CH3 CH3 H