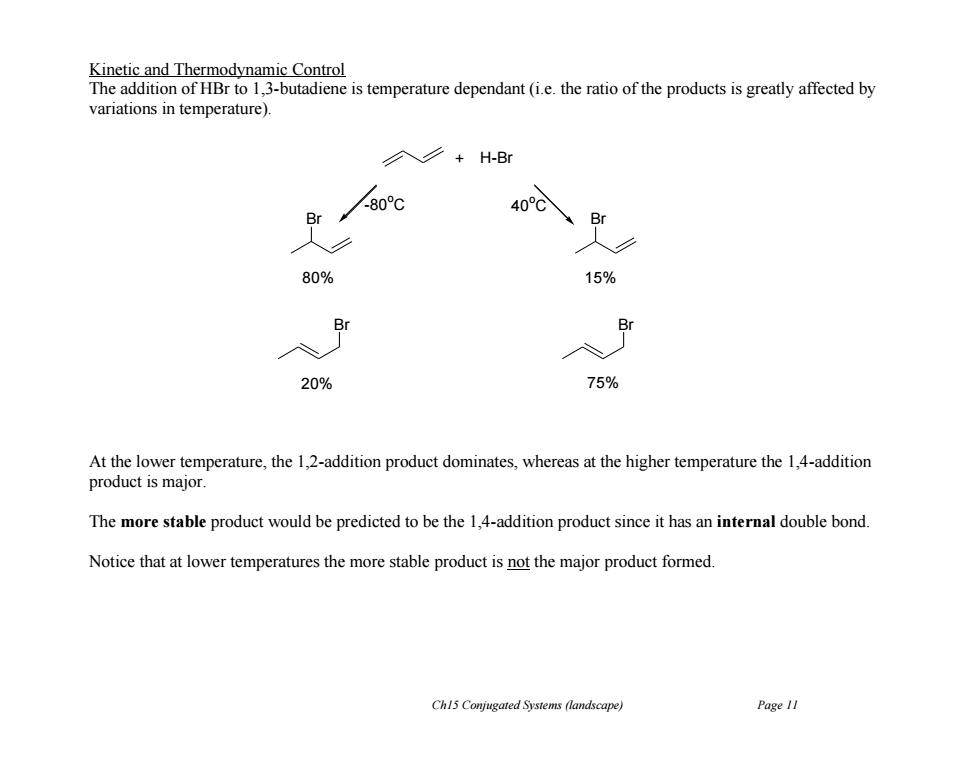
Kinetic and Thermodynamic Control The addition of HBr to 1,3-butadiene is temperature dependant(ie.the ratio of the products is greatly affected by variations in temperature). 入∠+H-Br 80c 40C Br Br 80% 15% Br Br 20% 75% At the lower temperature,the 1,2-addition product dominates,whereas at the higher temperature the 1,4-addition product is major. The more stable product would be predicted to be the 1,4-addition product since it has an internal double bond. Notice that at lower temperatures the more stable product is not the major product formed. Ch15 Conjugated Systems (landscape) Page 11
Ch15 Conjugated Systems (landscape) Page 11 Kinetic and Thermodynamic Control The addition of HBr to 1,3-butadiene is temperature dependant (i.e. the ratio of the products is greatly affected by variations in temperature). At the lower temperature, the 1,2-addition product dominates, whereas at the higher temperature the 1,4-addition product is major. The more stable product would be predicted to be the 1,4-addition product since it has an internal double bond. Notice that at lower temperatures the more stable product is not the major product formed. H-Br Br Br 80% 20% + Br Br 15% 75% -80 oC 40 oC
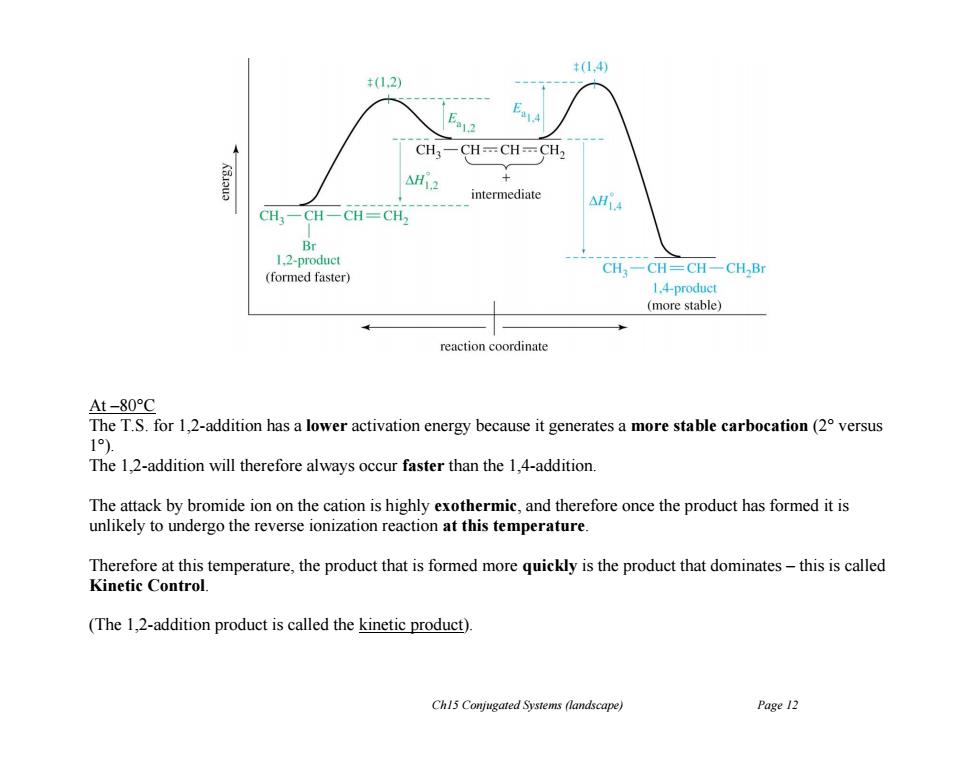
t(1.4) +(1.2) CH3-CH-CH=CH2 AH12 + intermediate △HiA CH一CH-CH=CH 1.2-product CH3一CH=CH一CH,Br (formed faster) 1.4-product (more stable) reaction coordinate At-80°C The T.S.for 1,2-addition has a lower activation energy because it generates a more stable carbocation(2 versus 1). The 1,2-addition will therefore always occur faster than the 1,4-addition. The attack by bromide ion on the cation is highly exothermic,and therefore once the product has formed it is unlikely to undergo the reverse ionization reaction at this temperature. Therefore at this temperature,the product that is formed more quickly is the product that dominates-this is called Kinetic Control. (The 1,2-addition product is called the kinetic product). Ch15 Conjugated Systems (landscape) Page 12
Ch15 Conjugated Systems (landscape) Page 12 At –80°C The T.S. for 1,2-addition has a lower activation energy because it generates a more stable carbocation (2° versus 1°). The 1,2-addition will therefore always occur faster than the 1,4-addition. The attack by bromide ion on the cation is highly exothermic, and therefore once the product has formed it is unlikely to undergo the reverse ionization reaction at this temperature. Therefore at this temperature, the product that is formed more quickly is the product that dominates – this is called Kinetic Control. (The 1,2-addition product is called the kinetic product)