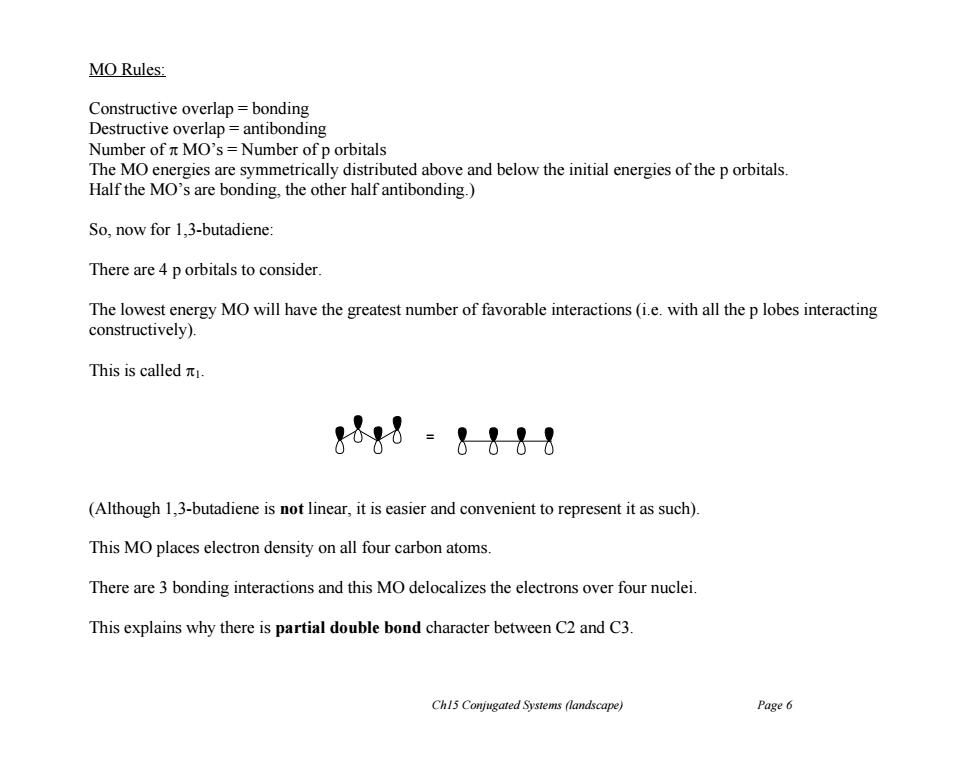
MO Rules: Constructive overlap bonding Destructive overlap=antibonding Number of n MO's=Number of p orbitals The MO energies are symmetrically distributed above and below the initial energies of the p orbitals Half the MO's are bonding,the other half antibonding.) So,now for 1.3-butadiene: There are 4 p orbitals to consider. The lowest energy MO will have the greatest number of favorable interactions (i.e.with all the p lobes interacting constructively). This is calledπ. &1&8 (Although 1,3-butadiene is not linear,it is easier and convenient to represent it as such). This MO places electron density on all four carbon atoms. There are 3 bonding interactions and this MO delocalizes the electrons over four nuclei. This explains why there is partial double bond character between C2 and C3. Ch15 Conjugated Systems (landscape) Page 6
Ch15 Conjugated Systems (landscape) Page 6 MO Rules: Constructive overlap = bonding Destructive overlap = antibonding Number of MO’s = Number of p orbitals The MO energies are symmetrically distributed above and below the initial energies of the p orbitals. Half the MO’s are bonding, the other half antibonding.) So, now for 1,3-butadiene: There are 4 p orbitals to consider. The lowest energy MO will have the greatest number of favorable interactions (i.e. with all the p lobes interacting constructively). This is called 1. (Although 1,3-butadiene is not linear, it is easier and convenient to represent it as such). This MO places electron density on all four carbon atoms. There are 3 bonding interactions and this MO delocalizes the electrons over four nuclei. This explains why there is partial double bond character between C2 and C3. =
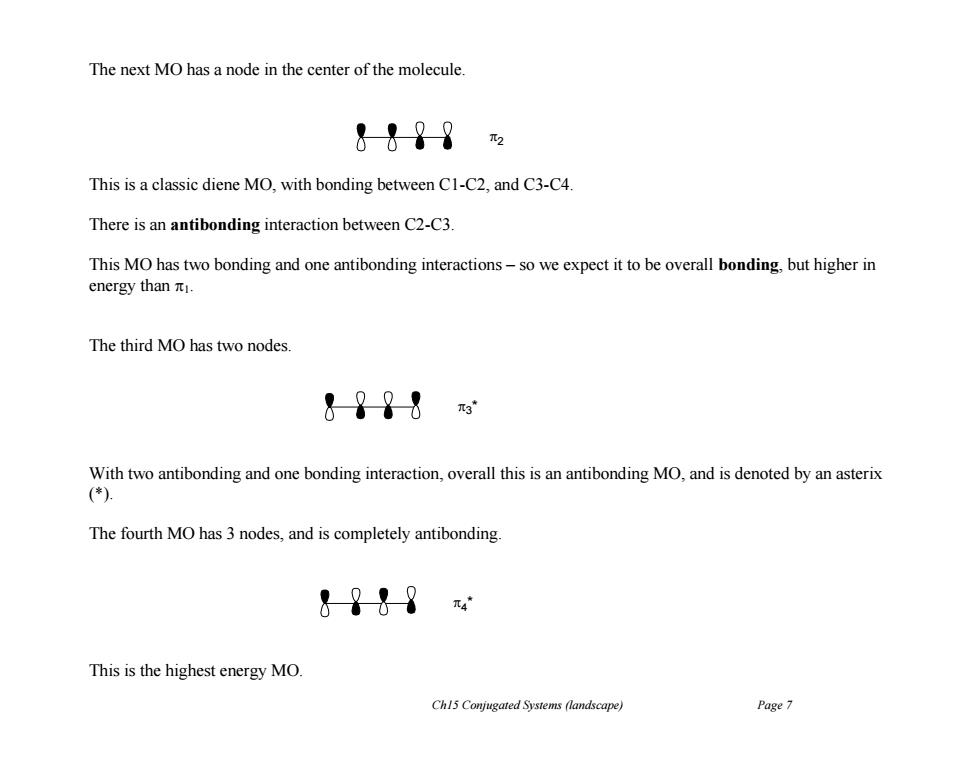
The next MO has a node in the center of the molecule. &&?8 This is a classic diene MO,with bonding between C1-C2,and C3-C4. There is an antibonding interaction between C2-C3. This MO has two bonding and one antibonding interactions-so we expect it to be overall bonding,but higher in energy than 1. The third MO has two nodes. &?98 With two antibonding and one bonding interaction,overall this is an antibonding MO,and is denoted by an asterix (*) The fourth MO has 3 nodes,and is completely antibonding &9t9 元4 This is the highest energy MO. Ch15 Conjugated Systems (landscape) Page 7
Ch15 Conjugated Systems (landscape) Page 7 The next MO has a node in the center of the molecule. This is a classic diene MO, with bonding between C1-C2, and C3-C4. There is an antibonding interaction between C2-C3. This MO has two bonding and one antibonding interactions – so we expect it to be overall bonding, but higher in energy than 1. The third MO has two nodes. With two antibonding and one bonding interaction, overall this is an antibonding MO, and is denoted by an asterix (*). The fourth MO has 3 nodes, and is completely antibonding. This is the highest energy MO. 2 3 * 4 *
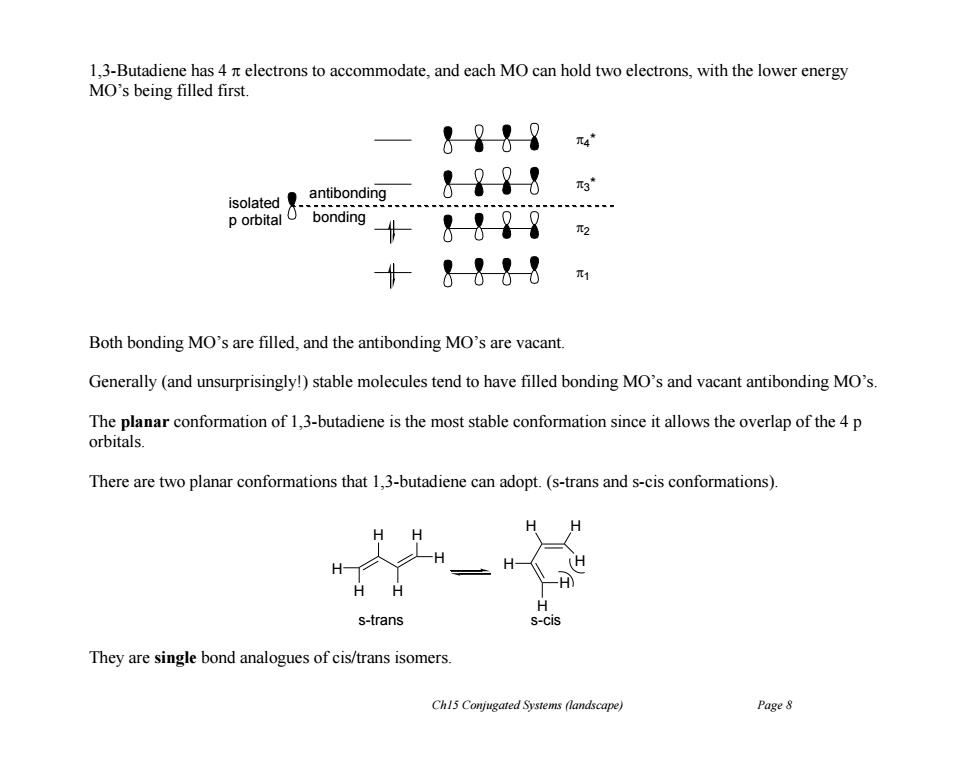
1,3-Butadiene has 4 n electrons to accommodate,and each MO can hold two electrons,with the lower energy MO's being filled first. &?↓9 antibonding &??8 isolated具. p orbital O bonding &-98 十&1-88m Both bonding MO's are filled,and the antibonding MO's are vacant Generally (and unsurprisingly!)stable molecules tend to have filled bonding MO's and vacant antibonding MO's. The planar conformation of 1,3-butadiene is the most stable conformation since it allows the overlap of the 4 p orbitals. There are two planar conformations that 1,3-butadiene can adopt.(s-trans and s-cis conformations). H (H H s-trans S-Cis They are single bond analogues of cis/trans isomers. Ch15 Conjugated Systems (landscape) Page 8
Ch15 Conjugated Systems (landscape) Page 8 1,3-Butadiene has 4 electrons to accommodate, and each MO can hold two electrons, with the lower energy MO’s being filled first. Both bonding MO’s are filled, and the antibonding MO’s are vacant. Generally (and unsurprisingly!) stable molecules tend to have filled bonding MO’s and vacant antibonding MO’s. The planar conformation of 1,3-butadiene is the most stable conformation since it allows the overlap of the 4 p orbitals. There are two planar conformations that 1,3-butadiene can adopt. (s-trans and s-cis conformations). They are single bond analogues of cis/trans isomers. 4 * 3 * 2 1 isolated p orbital antibonding bonding H H H H H H H H H H H H s-trans s-cis

The s-trans(single-trans)conformation is about 2.3kcal lower in energy than the s-cis conformation,which arises from the steric repulsions of the hydrogens. The barrier to rotation for these conformers is 4.9kcal This low energy difference means at room temperature these conformations are easily and rapidly interconverting. Allylic Cations Allylic cations are stabilized by resonance with the adjacent double bond,which delocalizes the positive charge over two carbon atoms. 入Br The delocalized cation can be represented by the two resonance structures or the combined structure. 1.2-and 1.4-Additions Allylic cations are often intermediates when there is electrophilic addition to conjugated dienes. Consider the case of electrophilic H-Br addition to 1.3-butadiene: Br Br H-Br 1,2-addition 1,4-addition 1,2-Addition and 1,4-addition refer to the relationship of the carbon atoms to which the H and Br are added. Ch15 Conjugated Systems (landscape) Page 9
Ch15 Conjugated Systems (landscape) Page 9 The s-trans (single-trans) conformation is about 2.3kcal lower in energy than the s-cis conformation, which arises from the steric repulsions of the hydrogens. The barrier to rotation for these conformers is 4.9kcal. This low energy difference means at room temperature these conformations are easily and rapidly interconverting. Allylic Cations Allylic cations are stabilized by resonance with the adjacent double bond, which delocalizes the positive charge over two carbon atoms. The delocalized cation can be represented by the two resonance structures or the combined structure. 1,2- and 1,4-Additions Allylic cations are often intermediates when there is electrophilic addition to conjugated dienes. Consider the case of electrophilic H-Br addition to 1,3-butadiene: 1,2-Addition and 1,4-addition refer to the relationship of the carbon atoms to which the H and Br are added. Br + -Br - + = 1 /2+ 1 /2+ H-Br Br Br 1,2-addition 1,4-addition
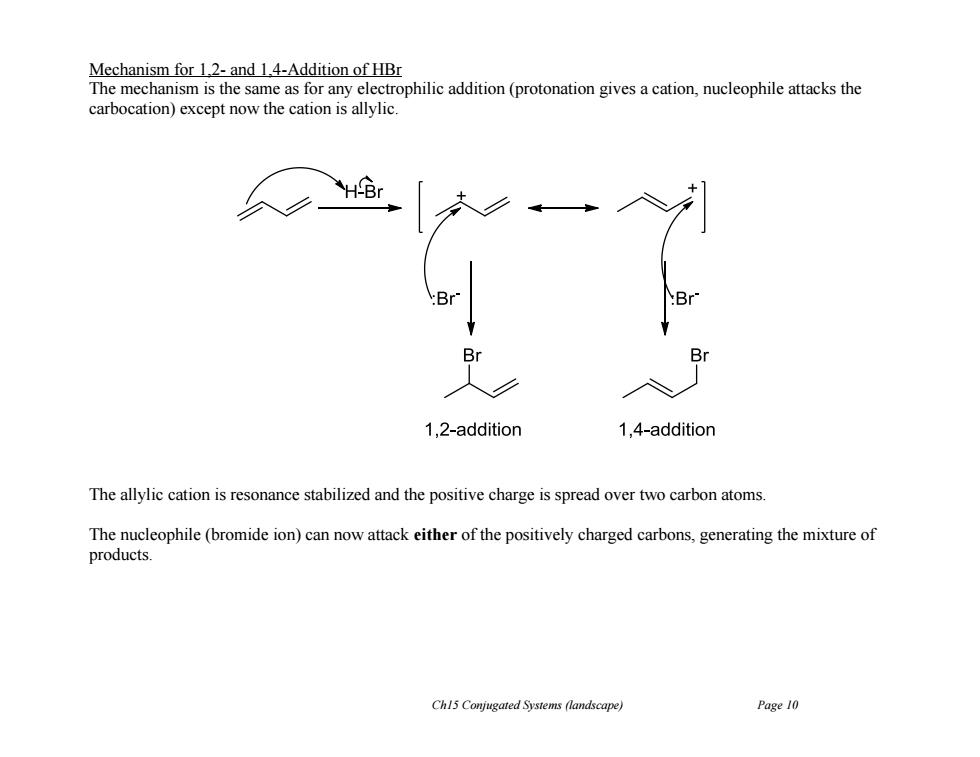
Mechanism for 1.2-and 1.4-Addition of HBr The mechanism is the same as for any electrophilic addition(protonation gives a cation,nucleophile attacks the carbocation)except now the cation is allylic. 1,2-addition 1,4-addition The allylic cation is resonance stabilized and the positive charge is spread over two carbon atoms. The nucleophile(bromide ion)can now attack either of the positively charged carbons,generating the mixture of products. Ch15 Conjugated Systems (landscape) Page 10
Ch15 Conjugated Systems (landscape) Page 10 Mechanism for 1,2- and 1,4-Addition of HBr The mechanism is the same as for any electrophilic addition (protonation gives a cation, nucleophile attacks the carbocation) except now the cation is allylic. The allylic cation is resonance stabilized and the positive charge is spread over two carbon atoms. The nucleophile (bromide ion) can now attack either of the positively charged carbons, generating the mixture of products