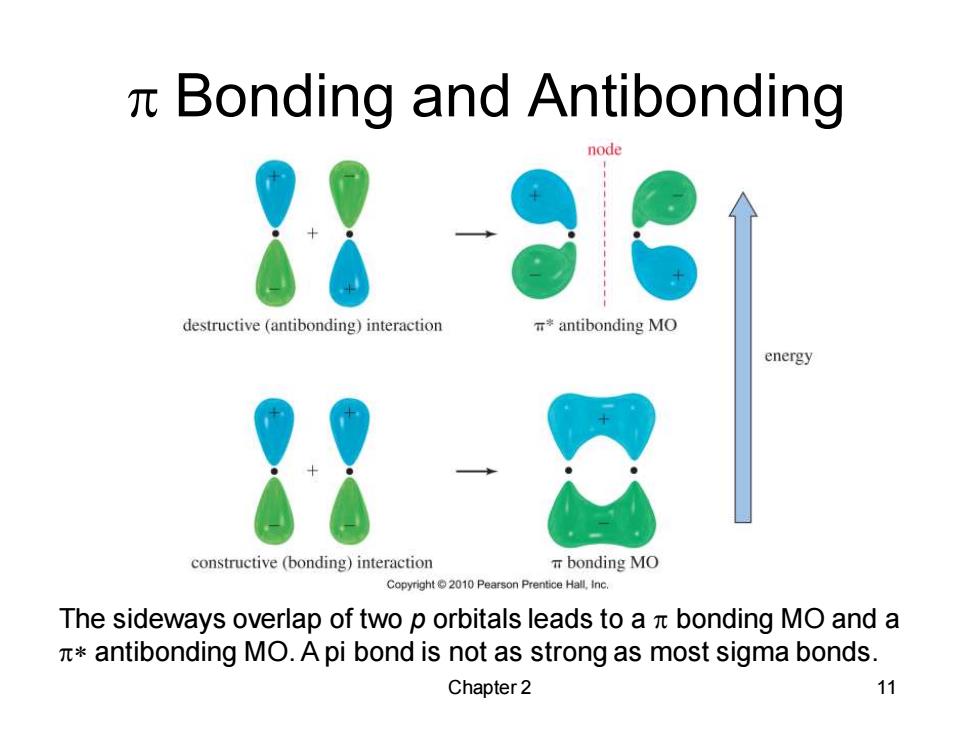
n Bonding and Antibonding node destructive (antibonding)interaction *antibonding MO energy constructive (bonding)interaction bonding MO Copyright 2010 Pearson Prentice Hall,Inc. The sideways overlap of two p orbitals leads to a n bonding MO and a antibonding MO.A pi bond is not as strong as most sigma bonds. Chapter 2 11
Chapter 2 11 p Bonding and Antibonding The sideways overlap of two p orbitals leads to a p bonding MO and a p* antibonding MO. A pi bond is not as strong as most sigma bonds

Multiple Bonds ·A double bond(2 pairs of shared electrons) half of m bond consists of a sigma bond and a pi bond. H1 o bond 1…H 。A triple bond(3 pairs H H of shared electrons) half of m bond consists of a sigma bond and two pi bonds. Chapter 2 12
Chapter 2 12 Multiple Bonds • A double bond (2 pairs of shared electrons) consists of a sigma bond and a pi bond. • A triple bond (3 pairs of shared electrons) consists of a sigma bond and two pi bonds
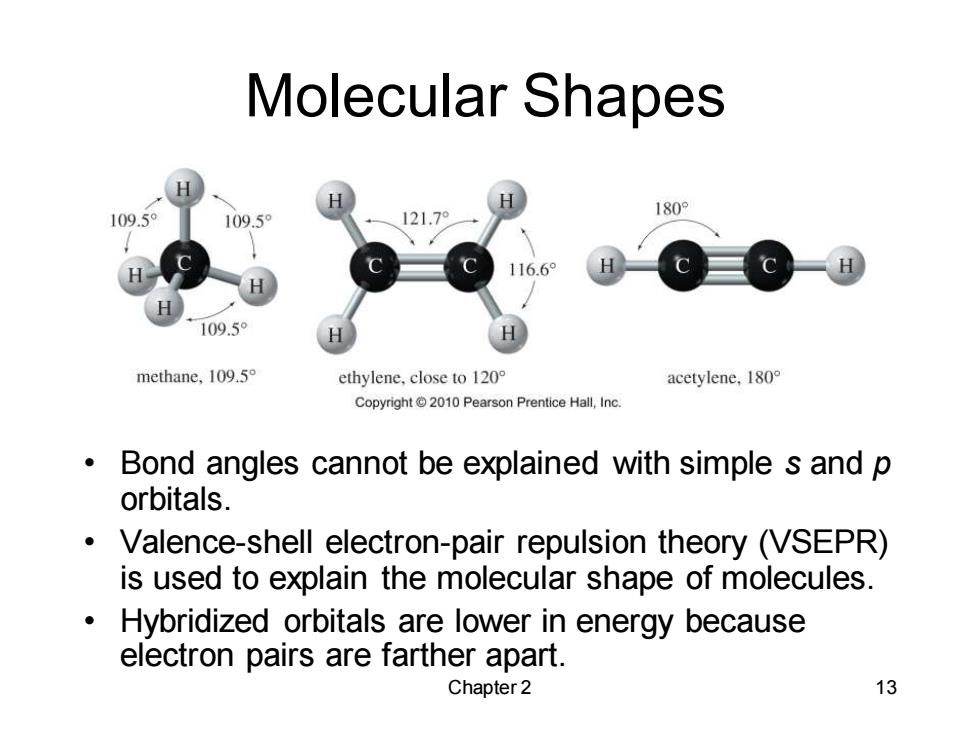
Molecular Shapes 109.5 180 109.5° 121.79 116.6 109.5 methane,109.5 ethylene,close to 120 acetylene,180 Copyright2010 Pearson Prentice Hall,Inc. Bond angles cannot be explained with simple s and p orbitals. Valence-shell electron-pair repulsion theory (VSEPR) is used to explain the molecular shape of molecules. Hybridized orbitals are lower in energy because electron pairs are farther apart. Chapter2 13
Chapter 2 13 Molecular Shapes • Bond angles cannot be explained with simple s and p orbitals. • Valence-shell electron-pair repulsion theory (VSEPR) is used to explain the molecular shape of molecules. • Hybridized orbitals are lower in energy because electron pairs are farther apart
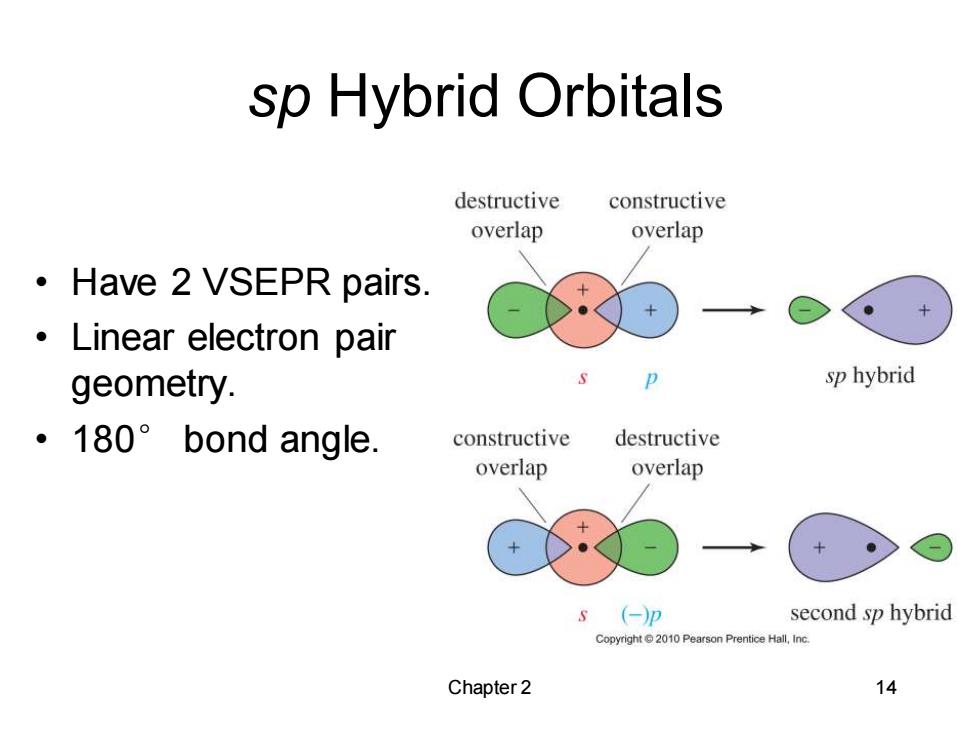
sp Hybrid Orbitals destructive constructive overlap overlap ·Have2 VSEPR pairs. 。Linear electron pair geometry. sp hybrid ·180°bond angle. constructive destructive overlap overlap s(-)p second sp hybrid Copyright2010 Pearson Prentice Hall,Inc. Chapter 2 14
Chapter 2 14 sp Hybrid Orbitals • Have 2 VSEPR pairs. • Linear electron pair geometry. • 180° bond angle
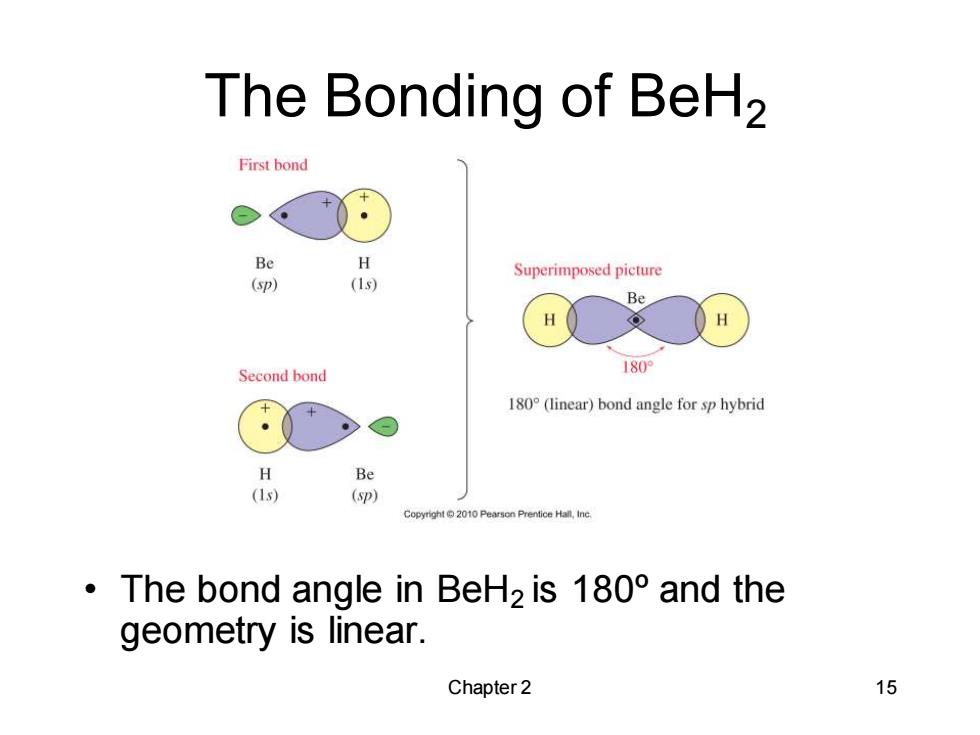
The Bonding of BeH2 First bond Be H Superimposed picture (p) (1s) Be H H Second bond 180 180(linear)bond angle for sp hybrid H Be (1s) (p) Copyright2010 Pearson Prentice Hall,Inc ·The bond angle in BeH2is180°and the geometry is linear. Chapter 2 15
Chapter 2 15 The Bonding of BeH2 • The bond angle in BeH2 is 180ºand the geometry is linear