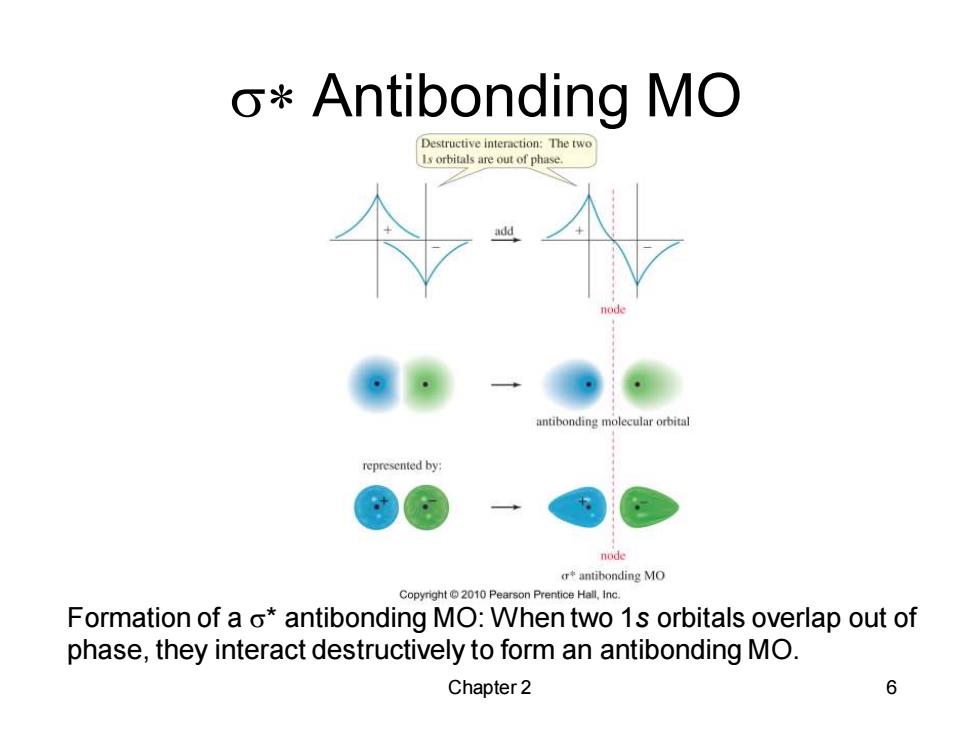
o*Antibonding MO Destructive interaction:The two Is orbitals are out of phase. antibonding molecular orbital represented by: node r本antibonding MO Copyright2010 Pearson Prentice Hall,Inc. Formation of a o*antibonding MO:When two 1s orbitals overlap out of phase,they interact destructively to form an antibonding MO. Chapter 2 6
Chapter 2 6 s* Antibonding MO Formation of a s* antibonding MO: When two 1s orbitals overlap out of phase, they interact destructively to form an antibonding MO
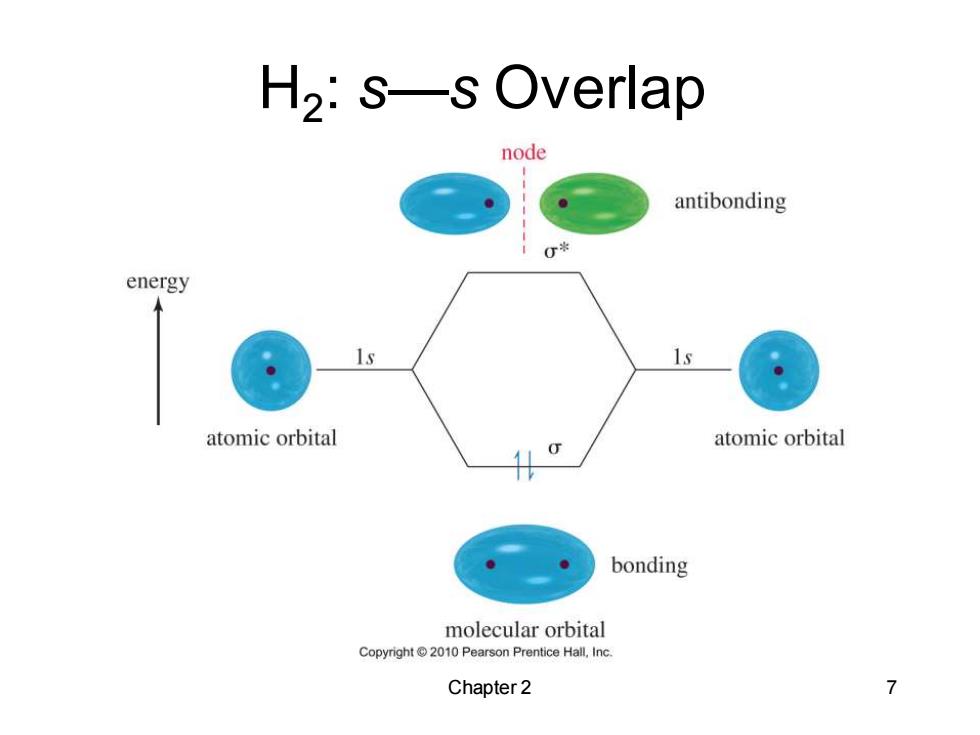
H2:s-s Overlap node antibonding energy 1s atomic orbital atomic orbital bonding molecular orbital Copyright2010 Pearson Prentice Hall,Inc. Chapter 2 7
Chapter 2 7 H2 : s—s Overlap
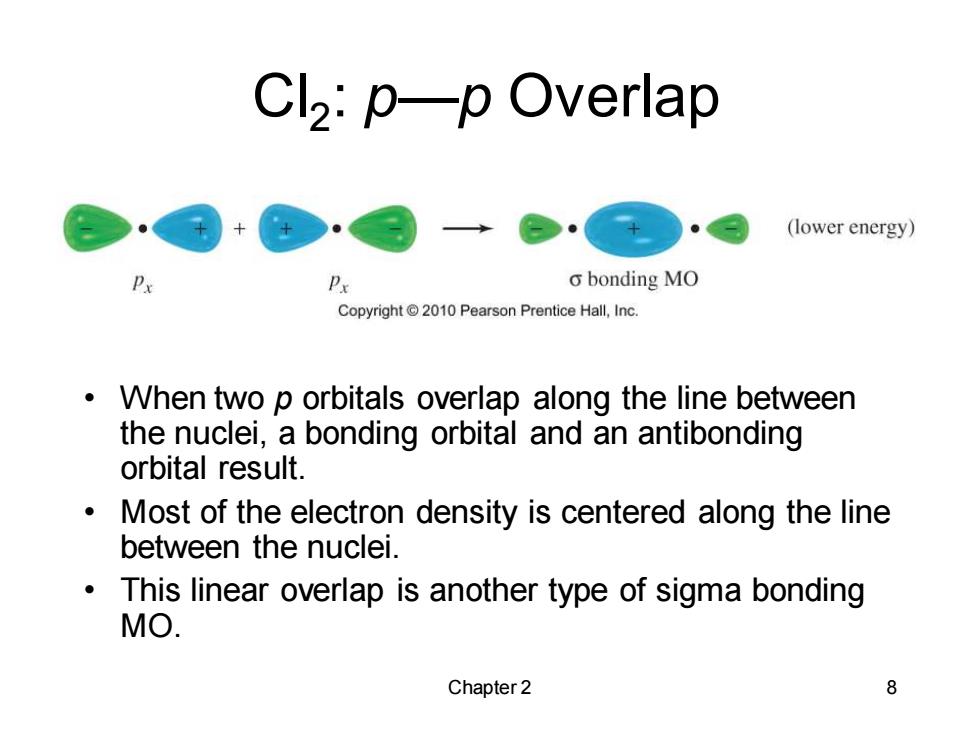
Cl2:p-p Overlap (lower energy) P Px o bonding MO Copyright 2010 Pearson Prentice Hall,Inc. When two p orbitals overlap along the line between the nuclei,a bonding orbital and an antibonding orbital result. Most of the electron density is centered along the line between the nuclei. This linear overlap is another type of sigma bonding MO. Chapter 2 8
Chapter 2 8 Cl2 : p—p Overlap • When two p orbitals overlap along the line between the nuclei, a bonding orbital and an antibonding orbital result. • Most of the electron density is centered along the line between the nuclei. • This linear overlap is another type of sigma bonding MO
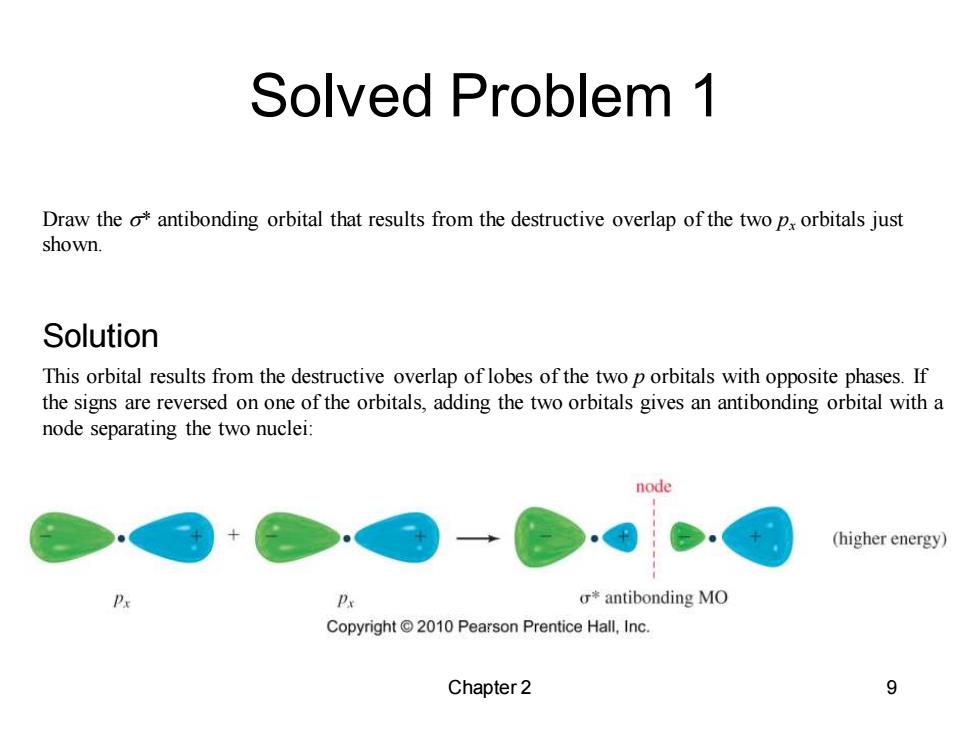
Solved Problem 1 Draw the o*antibonding orbital that results from the destructive overlap of the two px orbitals just shown. Solution This orbital results from the destructive overlap of lobes of the two p orbitals with opposite phases.If the signs are reversed on one of the orbitals,adding the two orbitals gives an antibonding orbital with a node separating the two nuclei: node (higher energy) Px o*antibonding MO Copyright 2010 Pearson Prentice Hall,Inc. Chapter 2 9
Solved Problem 1 Chapter 2 9 Draw the s* antibonding orbital that results from the destructive overlap of the two px orbitals just shown. This orbital results from the destructive overlap of lobes of the two p orbitals with opposite phases. If the signs are reversed on one of the orbitals, adding the two orbitals gives an antibonding orbital with a node separating the two nuclei: Solution

s and p Orbital Overlap (lower energy) o bonding MO node (higher energy) Px (-)5 σ*antibonding MO Copyright2010 Pearson Prentice Hall.Inc. Overlap of an s orbital with a p orbital also gives a bonding MO and an antibonding MO. Chapter 2 10
Chapter 2 10 s and p Orbital Overlap • Overlap of an s orbital with a p orbital also gives a bonding MO and an antibonding MO