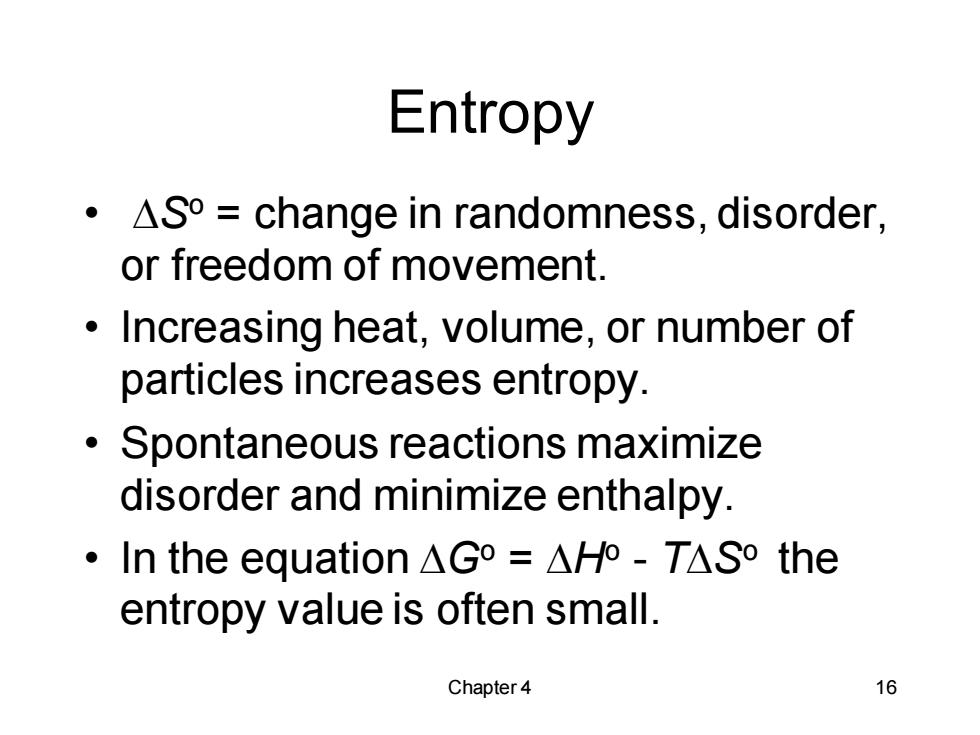
Entropy ·△So=change in randomness,disorder, or freedom of movement. Increasing heat,volume,or number of particles increases entropy. Spontaneous reactions maximize disorder and minimize enthalpy. ·In the equation△Go=△Ho-T△So the entropy value is often small. Chapter 4 16
Chapter 4 16 Entropy • DSo = change in randomness, disorder, or freedom of movement. • Increasing heat, volume, or number of particles increases entropy. • Spontaneous reactions maximize disorder and minimize enthalpy. • In the equation DGo = DHo - TDSo the entropy value is often small

Solved Problem 1 Calculate the value of A G for the chlorination of methane. Solution △G°=-2.303 RT(log Kea) Keg for the chlorination is 1.1 x 1019,and log Keg =19.04 At 25C(about 298 Kelvin),the value of RT is RT=(8.314 J/kelvin-mol)(298 kelvins)=2478 J/mol,or 2.48 kJ/mol Substituting,we have △G°=(-2.303)2.478kJ/mol)19.04)=-108.7kJ/mol(-25.9kcal>mol) This is a large negative value for A Go,showing that this chlorination has a large driving force that pushes it toward completion. Chapter 4 17
Chapter 4 17 Calculate the value of D G° for the chlorination of methane. D G° = –2.303RT(log Keq) Keq for the chlorination is 1.1 x 1019, and log Keq = 19.04 At 25 °C (about 298 ° Kelvin), the value of RT is RT = (8.314 J/kelvin-mol)(298 kelvins) = 2478 J/mol, or 2.48 kJ/mol Substituting, we have D G° = (–2.303)(2.478 kJ/mol)(19.04) = –108.7 kJ/mol (–25.9 kcal>mol) This is a large negative value for D G°, showing that this chlorination has a large driving force that pushes it toward completion. Solved Problem 1 Solution
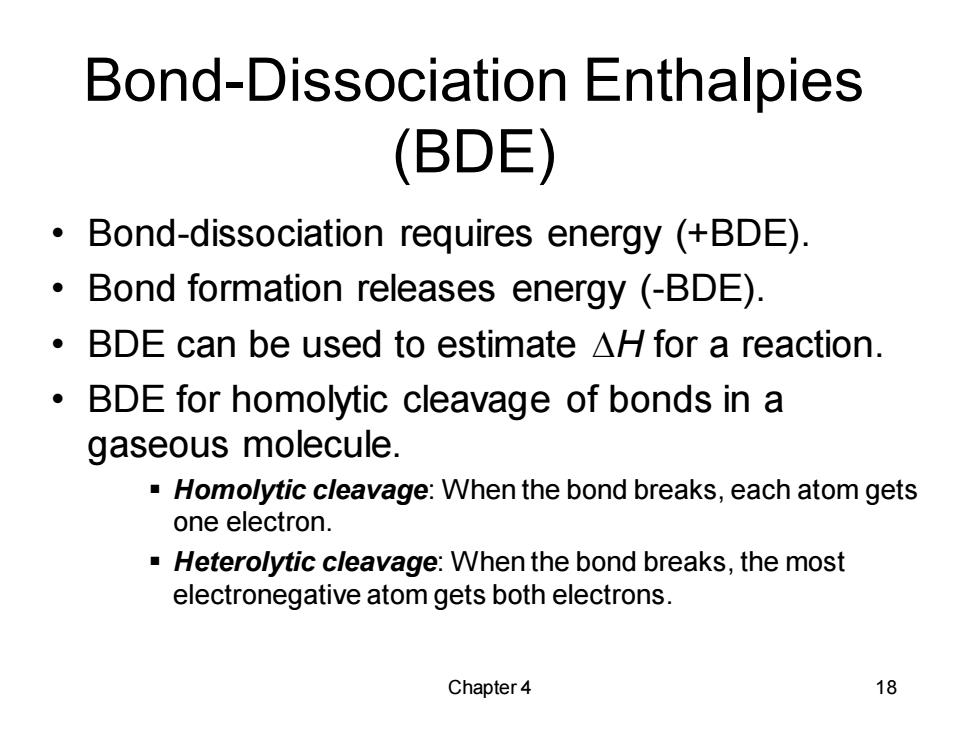
Bond-Dissociation Enthalpies (BDE) Bond-dissociation requires energy (+BDE). Bond formation releases energy (-BDE). ·BDE can be used to estimate△Hfor a reaction. 。 BDE for homolytic cleavage of bonds in a gaseous molecule. -Homolytic cleavage:When the bond breaks,each atom gets one electron. Heterolytic cleavage:When the bond breaks,the most electronegative atom gets both electrons. Chapter 4 18
Chapter 4 18 Bond-Dissociation Enthalpies (BDE) • Bond-dissociation requires energy (+BDE). • Bond formation releases energy (-BDE). • BDE can be used to estimate DH for a reaction. • BDE for homolytic cleavage of bonds in a gaseous molecule. ▪ Homolytic cleavage: When the bond breaks, each atom gets one electron. ▪ Heterolytic cleavage: When the bond breaks, the most electronegative atom gets both electrons