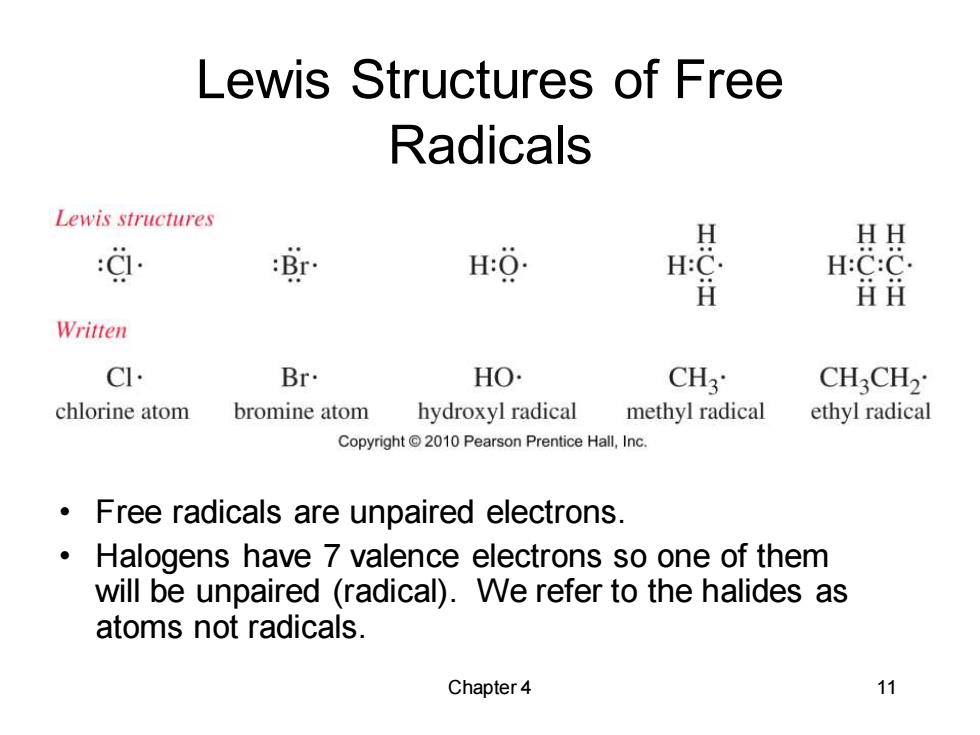
Lewis Structures of Free Radicals Lewis structures 过 涂 H HH H: H:C. H:C:C. ⅱi Written C Br. HO. CH3 CHCH2 chlorine atom bromine atom hydroxyl radical methyl radical ethyl radical Copyright2010 Pearson Prentice Hall,Inc. Free radicals are unpaired electrons. Halogens have 7 valence electrons so one of them will be unpaired (radical).We refer to the halides as atoms not radicals. Chapter 4 11
Chapter 4 11 Lewis Structures of Free Radicals • Free radicals are unpaired electrons. • Halogens have 7 valence electrons so one of them will be unpaired (radical). We refer to the halides as atoms not radicals
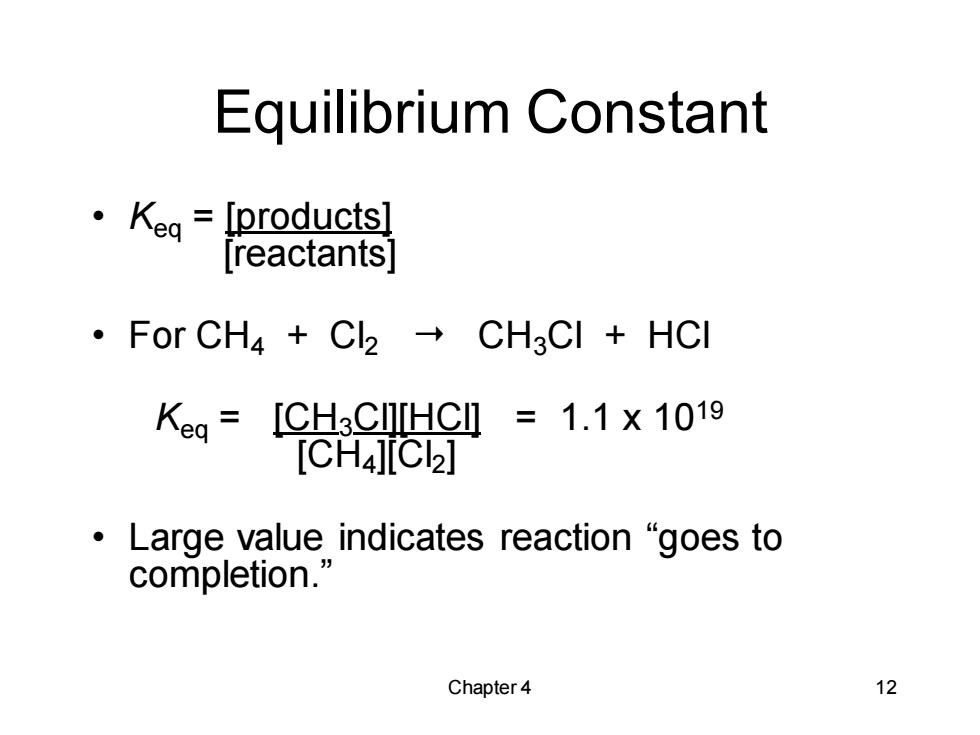
Equilibrium Constant ·Keg=[products] [reactants] ·For CH4+C2→CH3CI+HCl Keg=[CHC[HC=1.1×1019 [CH4][c2] 。Large value indicates reaction“goes to completion.' Chapter 4 12
Chapter 4 12 Equilibrium Constant • Keq = [products] [reactants] • For CH4 + Cl2 CH3Cl + HCl Keq = [CH3Cl][HCl] = 1.1 x 1019 [CH4][Cl2] • Large value indicates reaction “goes to completion
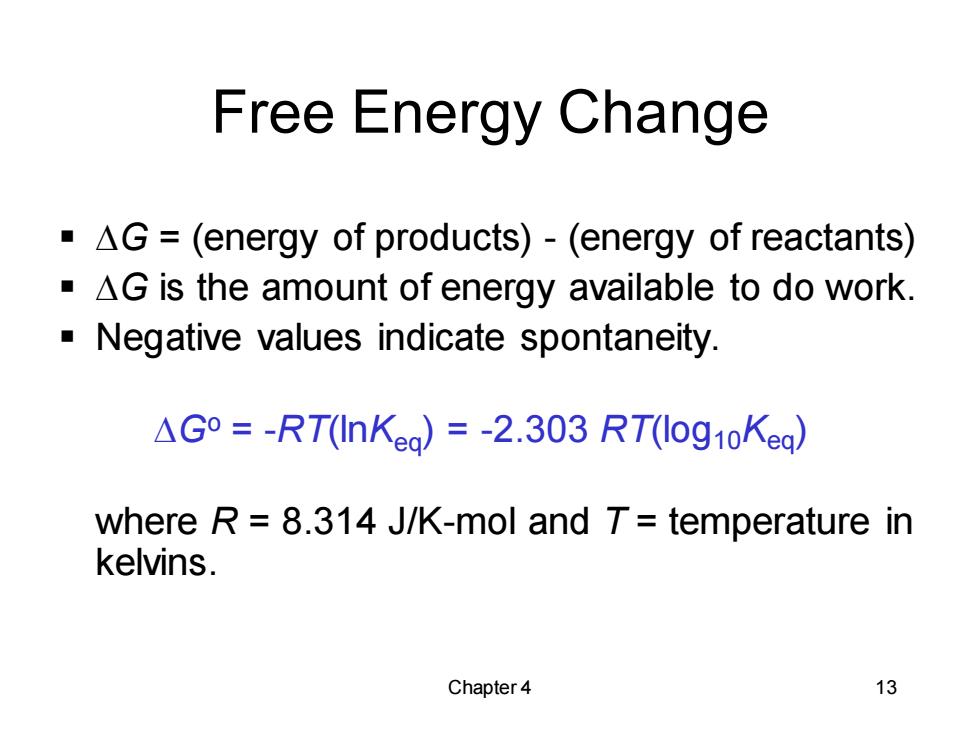
Free Energy Change AG =(energy of products)-(energy of reactants) AG is the amount of energy available to do work. Negative values indicate spontaneity △G°=-RT(In Kea)=-2.303RT(log1oKea) where R=8.314 J/K-mol and T=temperature in kelvins. Chapter4 13
Chapter 4 13 Free Energy Change ▪ DG = (energy of products) - (energy of reactants) ▪ DG is the amount of energy available to do work. ▪ Negative values indicate spontaneity. DGo = -RT(lnKeq) = -2.303 RT(log10Keq) where R = 8.314 J/K-mol and T = temperature in kelvins
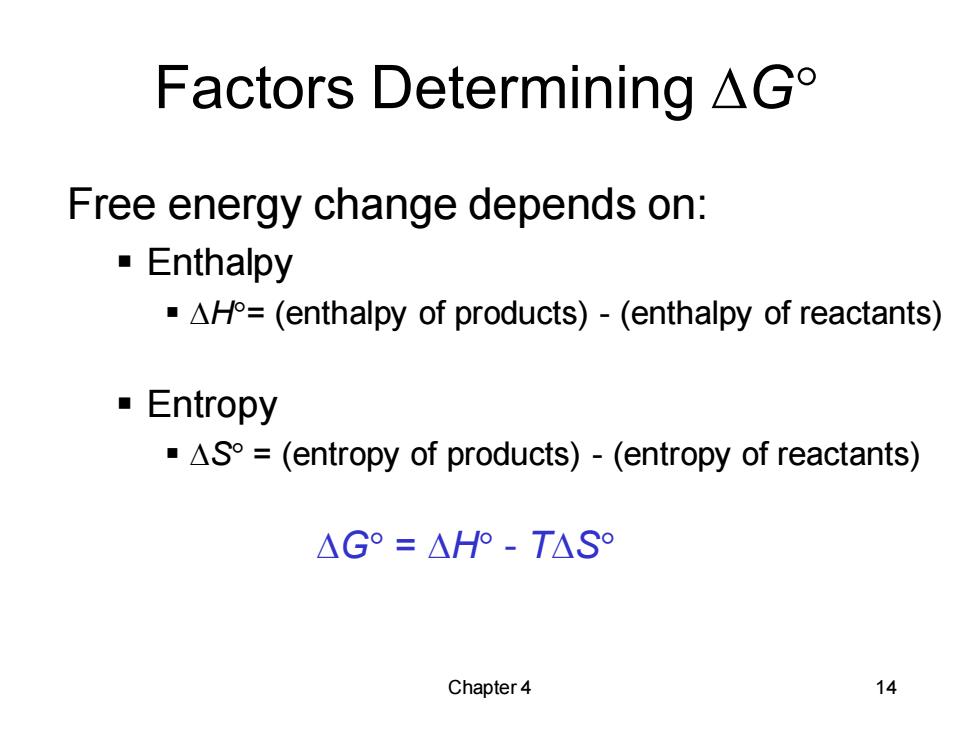
Factors Determining AGo Free energy change depends on: Enthalpy AH=(enthalpy of products)-(enthalpy of reactants) Entropy AS=(entropy of products)-(entropy of reactants) △G°=△HP-T△S° Chapter 4 14
Chapter 4 14 Factors Determining DG Free energy change depends on: ▪ Enthalpy ▪ DH= (enthalpy of products) - (enthalpy of reactants) ▪ Entropy ▪ DS = (entropy of products) - (entropy of reactants) DG = DH - TDS
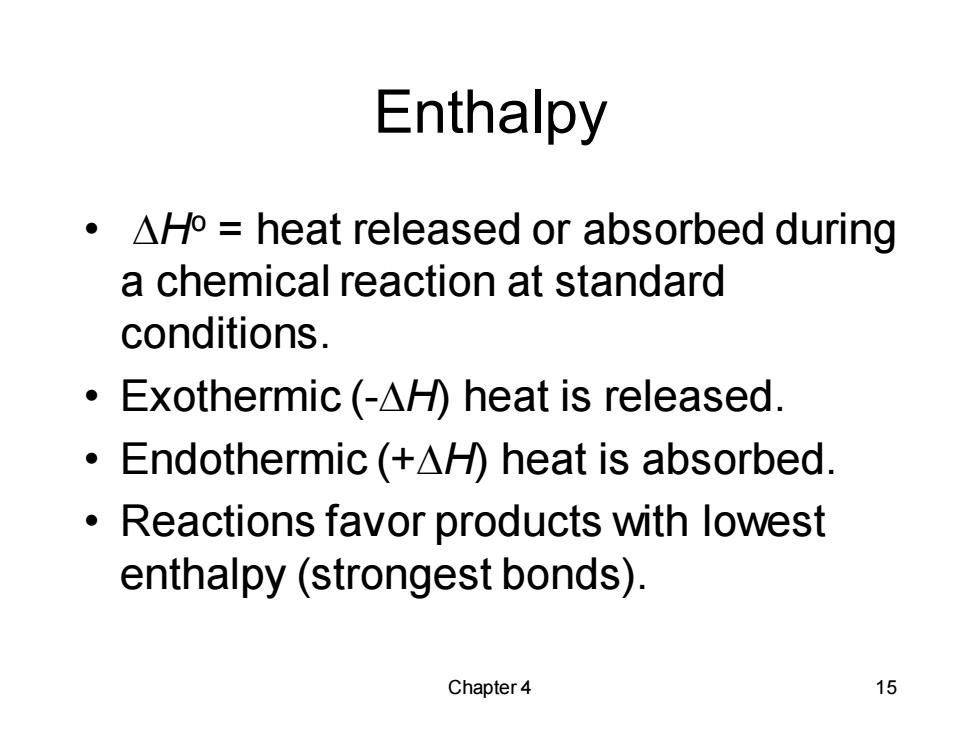
Enthalpy ·△Ho=heat released or absorbed during a chemical reaction at standard conditions. ·Exothermic(-△H heat is released. ·Endothermic(+△)heat is absorbed. Reactions favor products with lowest enthalpy(strongest bonds). Chapter 4 15
Chapter 4 15 Enthalpy • DHo = heat released or absorbed during a chemical reaction at standard conditions. • Exothermic (-DH) heat is released. • Endothermic (+DH) heat is absorbed. • Reactions favor products with lowest enthalpy (strongest bonds)