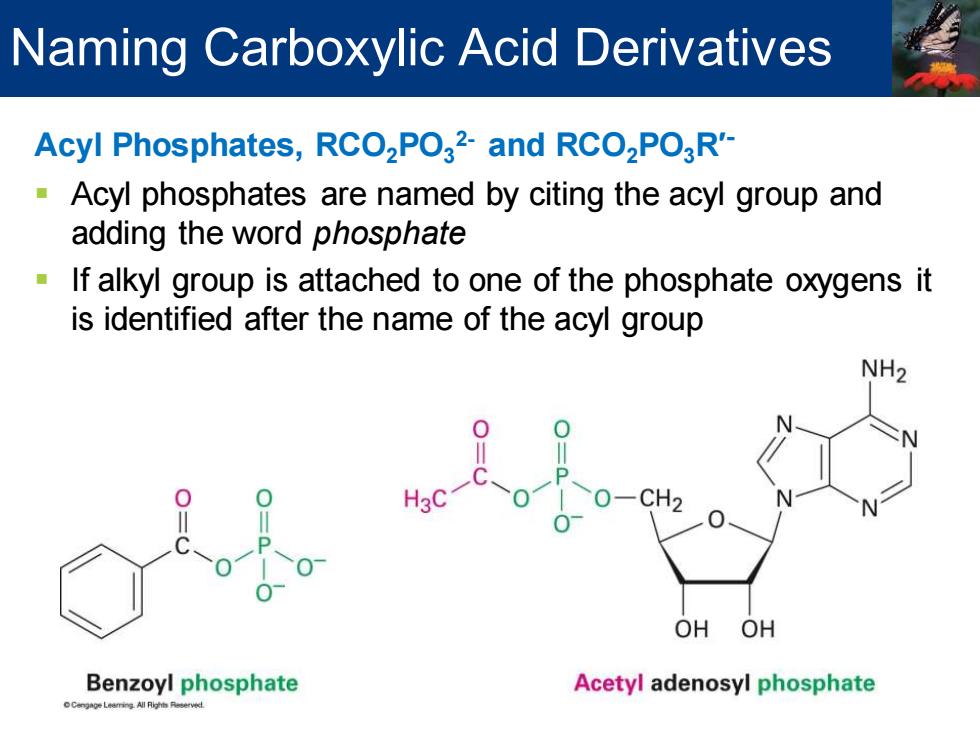
Naming Carboxylic Acid Derivatives Acyl Phosphates,RCO2PO32-and RCO2PO3R'- Acyl phosphates are named by citing the acyl group and adding the word phosphate If alkyl group is attached to one of the phosphate oxygens it is identified after the name of the acyl group NH2 H3C 0-CH2 OH OH Benzoyl phosphate Acetyl adenosyl phosphate
Acyl Phosphates, RCO2PO3 2- and RCO2PO3R′- ▪ Acyl phosphates are named by citing the acyl group and adding the word phosphate ▪ If alkyl group is attached to one of the phosphate oxygens it is identified after the name of the acyl group Naming Carboxylic Acid Derivatives
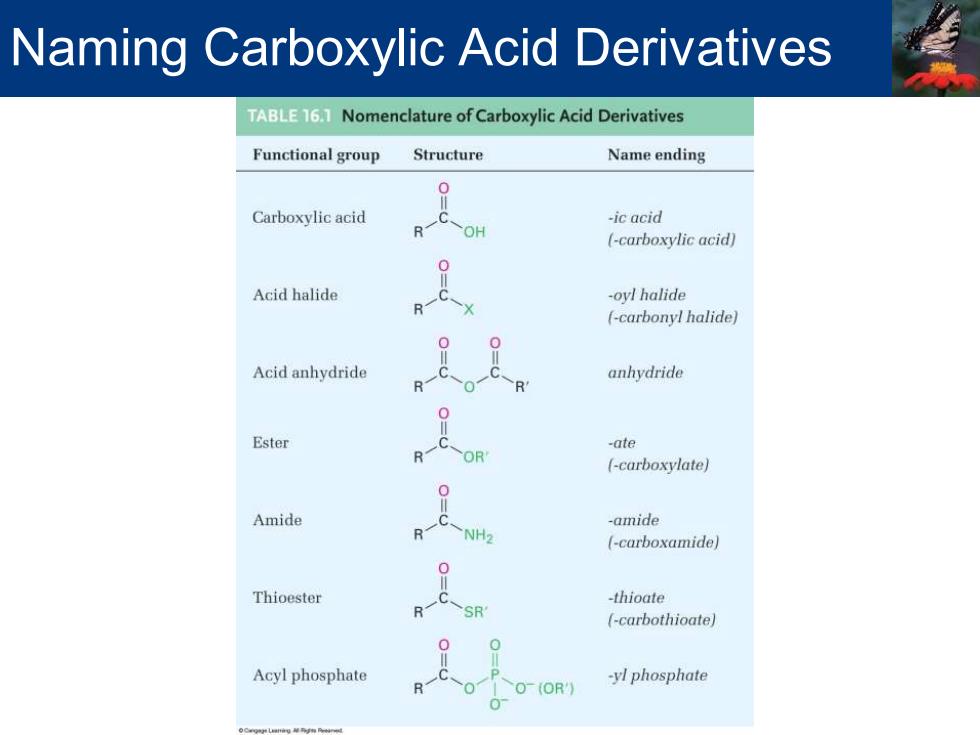
Naming Carboxylic Acid Derivatives TABLE15 Nomenclature of Carboxylic Acid Derivatives Functional group Structure Name ending Carboxylic acid -ic acid (-carboxylic acid) Acid halide -oyl halide (-carbonyl halide) Acid anhydride anhydride Ester -ate (-carboxylate) Amide -amide (-carboxamide) Thioester -thioate (-carbothioate) Acyl phosphate -yl phosphate (OR
Naming Carboxylic Acid Derivatives
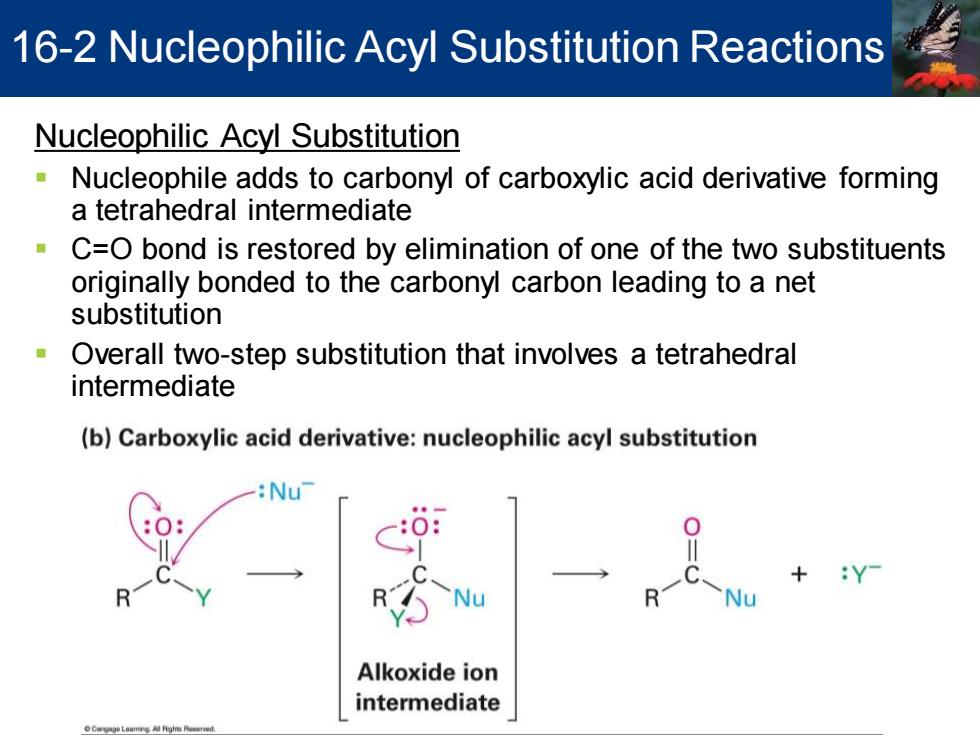
16-2 Nucleophilic Acyl Substitution Reactions Nucleophilic Acyl Substitution Nucleophile adds to carbonyl of carboxylic acid derivative forming a tetrahedral intermediate C=O bond is restored by elimination of one of the two substituents originally bonded to the carbonyl carbon leading to a net substitution Overall two-step substitution that involves a tetrahedral intermediate (b)Carboxylic acid derivative:nucleophilic acyl substitution Nu Alkoxide ion intermediate
Nucleophilic Acyl Substitution ▪ Nucleophile adds to carbonyl of carboxylic acid derivative forming a tetrahedral intermediate ▪ C=O bond is restored by elimination of one of the two substituents originally bonded to the carbonyl carbon leading to a net substitution ▪ Overall two-step substitution that involves a tetrahedral intermediate 16-2 Nucleophilic Acyl Substitution Reactions
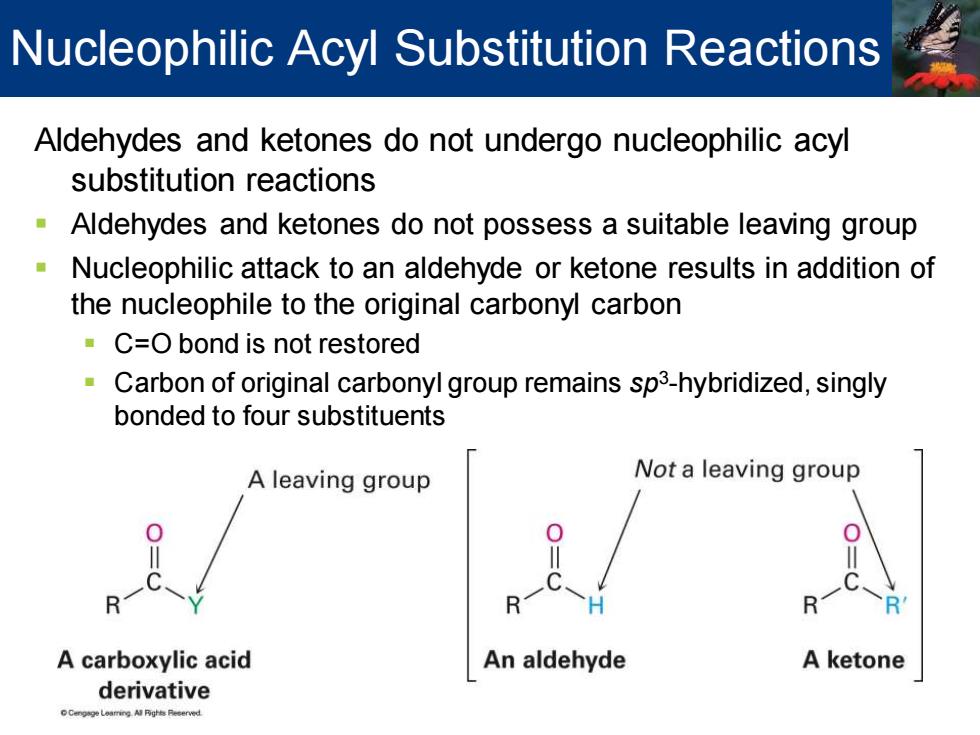
Nucleophilic Acyl Substitution Reactions Aldehydes and ketones do not undergo nucleophilic acyl substitution reactions Aldehydes and ketones do not possess a suitable leaving group Nucleophilic attack to an aldehyde or ketone results in addition of the nucleophile to the original carbonyl carbon C=O bond is not restored Carbon of original carbonyl group remains sp3-hybridized,singly bonded to four substituents A leaving group Not a leaving group A carboxylic acid An aldehyde A ketone derivative
Aldehydes and ketones do not undergo nucleophilic acyl substitution reactions ▪ Aldehydes and ketones do not possess a suitable leaving group ▪ Nucleophilic attack to an aldehyde or ketone results in addition of the nucleophile to the original carbonyl carbon ▪ C=O bond is not restored ▪ Carbon of original carbonyl group remains sp3 -hybridized, singly bonded to four substituents Nucleophilic Acyl Substitution Reactions

Nucleophilic Acyl Substitution Reactions Relative reactivity of carboxylic acid derivatives Both addition and elimination steps affect the overall rate of nucleophilic acyl substitution reaction Addition step is generally rate-limiting due to steric and electronic factors Sterically unhindered acid derivatives are more accessible to approaching nucleophile R N R R R R R H Reactivity gnge Leaming All Righes Peservd
Relative reactivity of carboxylic acid derivatives ▪ Both addition and elimination steps affect the overall rate of nucleophilic acyl substitution reaction ▪ Addition step is generally rate-limiting due to steric and electronic factors ▪ Sterically unhindered acid derivatives are more accessible to approaching nucleophile Nucleophilic Acyl Substitution Reactions