
6-3 Radical Reactions Radical Highly reactive because it contains an atom with an odd number of electrons (usually seven)in a valence shell Can achieve a valence shell octet through: Radical substitution reaction a Radical abstracts an atom and one bonding electron from another reactant Unpaired Unpaired electron electron Rad. A: Rad:A ·B Reactant Substitution Product radical product radical
Radical ▪ Highly reactive because it contains an atom with an odd number of electrons (usually seven) in a valence shell ▪ Can achieve a valence shell octet through: ▪ Radical substitution reaction ▪ Radical abstracts an atom and one bonding electron from another reactant 6-3 Radical Reactions
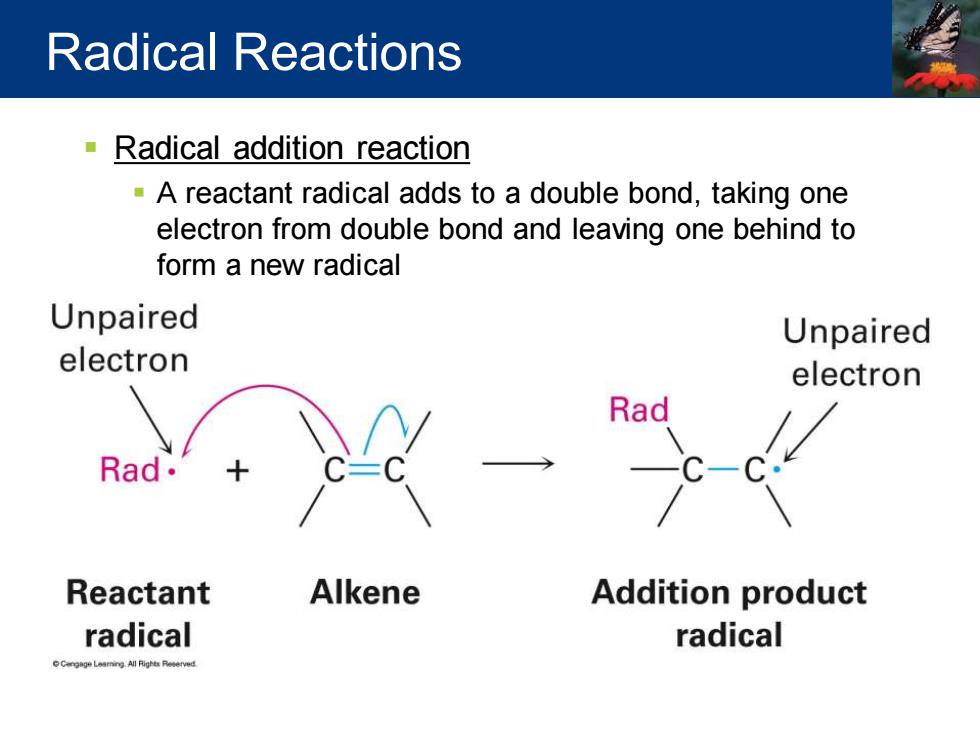
Radical Reactions Radical addition reaction -A reactant radical adds to a double bond,taking one electron from double bond and leaving one behind to form a new radical Unpaired Unpaired electron electron Reactant Alkene Addition product radical radical
▪ Radical addition reaction ▪ A reactant radical adds to a double bond, taking one electron from double bond and leaving one behind to form a new radical Radical Reactions
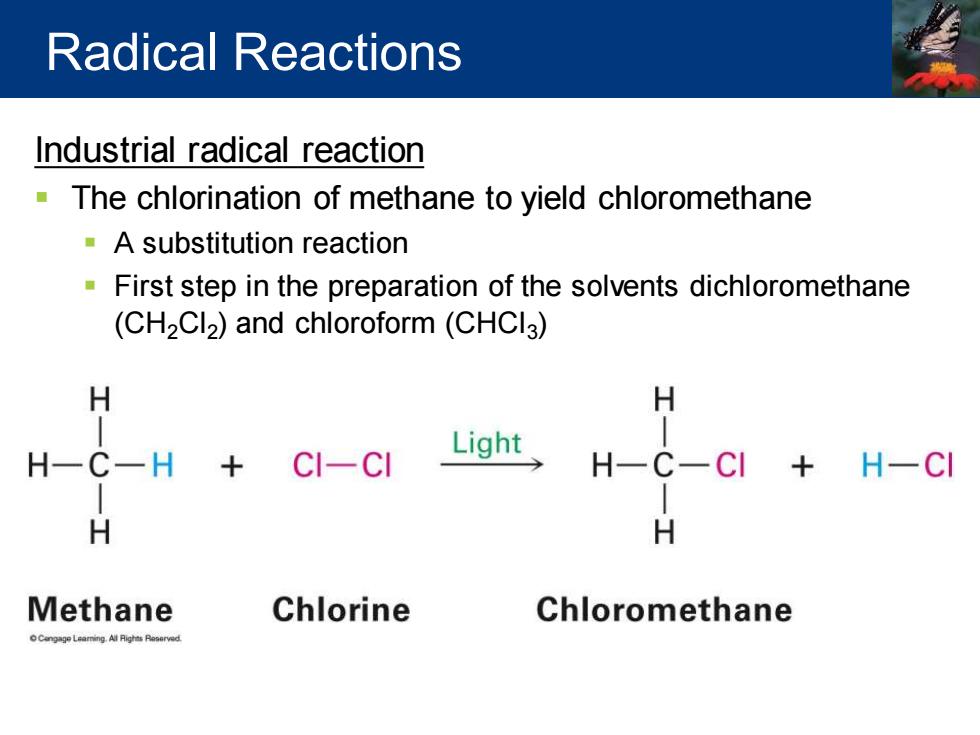
Radical Reactions Industrial radical reaction The chlorination of methane to yield chloromethane A substitution reaction First step in the preparation of the solvents dichloromethane (CH2Cl2)and chloroform(CHCI3) H H Light H -H CI-CI H H-CI H H Methane Chlorine Chloromethane
Industrial radical reaction ▪ The chlorination of methane to yield chloromethane ▪ A substitution reaction ▪ First step in the preparation of the solvents dichloromethane (CH2Cl2 ) and chloroform (CHCl3 ) Radical Reactions
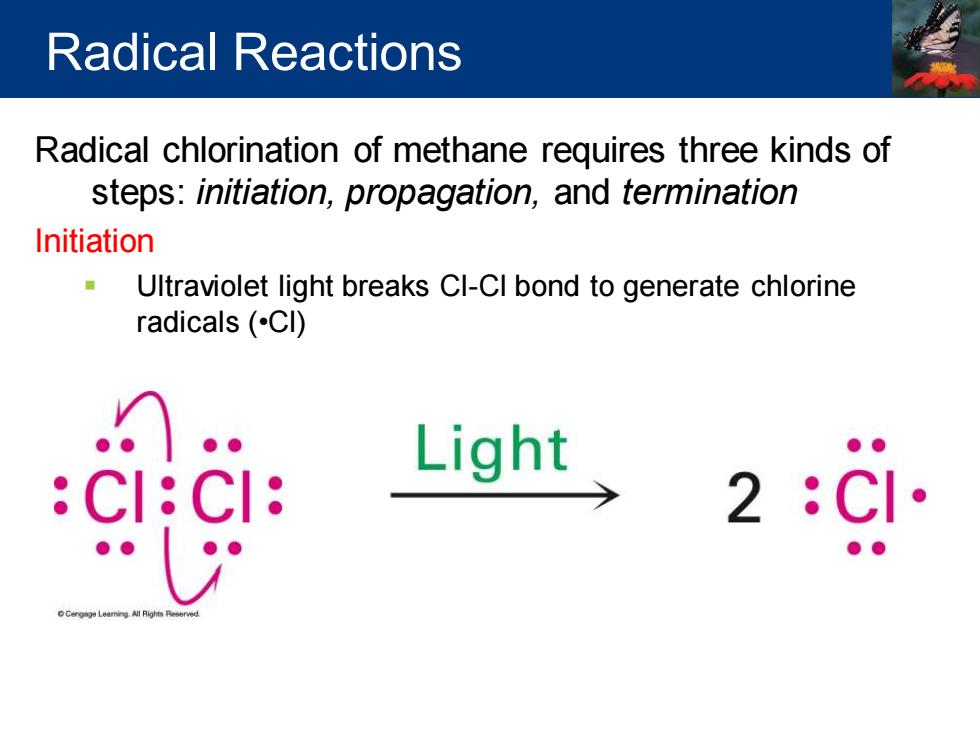
Radical Reactions Radical chlorination of methane requires three kinds of steps:initiation,propagation,and termination Initiation Ultraviolet light breaks Cl-CI bond to generate chlorine radicals(CI) CI: Light 2 CI
Radical chlorination of methane requires three kinds of steps: initiation, propagation, and termination Initiation ▪ Ultraviolet light breaks Cl-Cl bond to generate chlorine radicals (•Cl) Radical Reactions
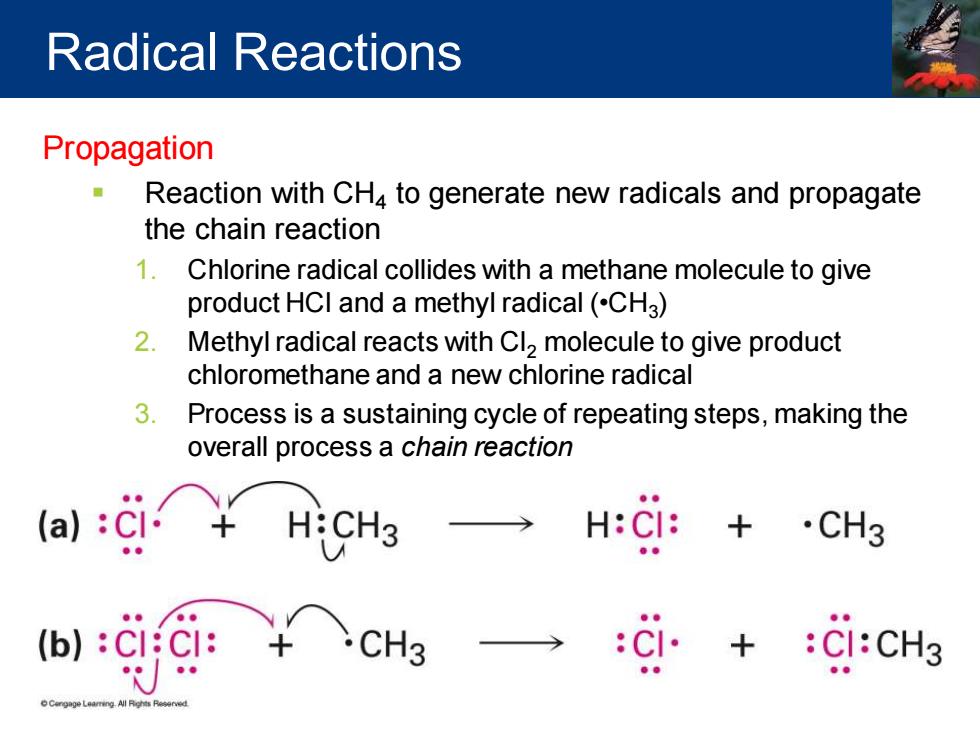
Radical Reactions Propagation Reaction with CH4 to generate new radicals and propagate the chain reaction 1.Chlorine radical collides with a methane molecule to give product HCI and a methyl radical(CHa) 2.Methyl radical reacts with Cl2 molecule to give product chloromethane and a new chlorine radical 3.Process is a sustaining cycle of repeating steps,making the overall process a chain reaction .CH3 (b):C:C:
Propagation ▪ Reaction with CH4 to generate new radicals and propagate the chain reaction 1. Chlorine radical collides with a methane molecule to give product HCl and a methyl radical (•CH3 ) 2. Methyl radical reacts with Cl2 molecule to give product chloromethane and a new chlorine radical 3. Process is a sustaining cycle of repeating steps, making the overall process a chain reaction Radical Reactions