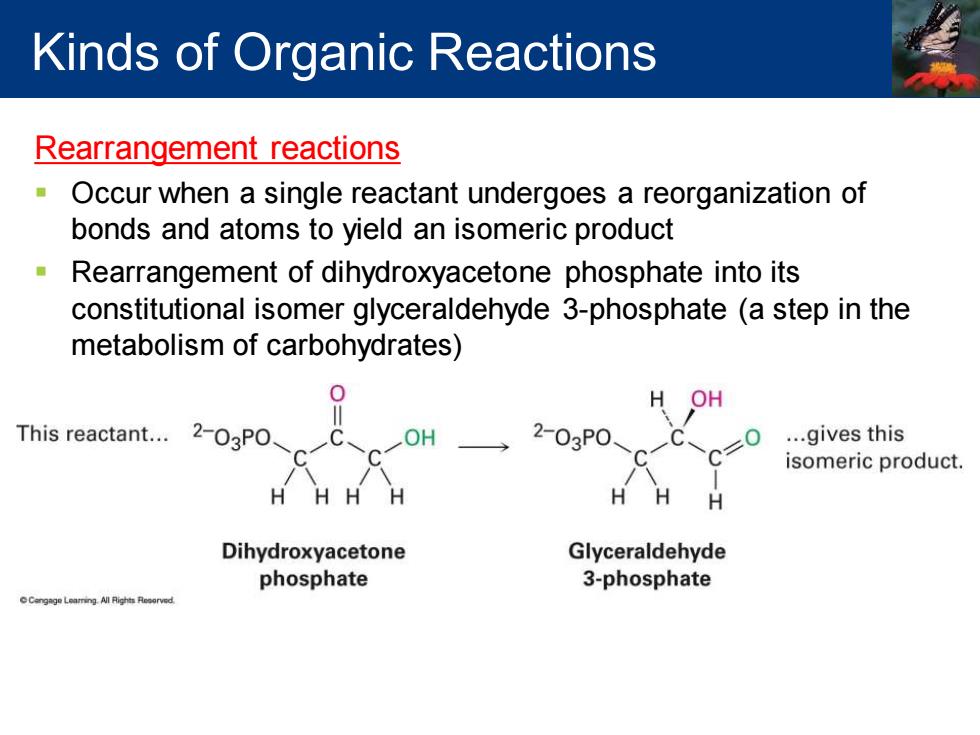
Kinds of Organic Reactions Rearrangement reactions n Occur when a single reactant undergoes a reorganization of bonds and atoms to yield an isomeric product Rearrangement of dihydroxyacetone phosphate into its constitutional isomer glyceraldehyde 3-phosphate (a step in the metabolism of carbohydrates) H OH This reactant... 2-03P0 OH 2-03P0 ...gives this isomeric product. H HH Dihydroxyacetone Glyceraldehyde phosphate 3-phosphate
Rearrangement reactions ▪ Occur when a single reactant undergoes a reorganization of bonds and atoms to yield an isomeric product ▪ Rearrangement of dihydroxyacetone phosphate into its constitutional isomer glyceraldehyde 3-phosphate (a step in the metabolism of carbohydrates) Kinds of Organic Reactions
6-2 How Organic Reactions Occur: Mechanisms Reaction Mechanism An overall description of how a reaction occurs at each stage of a chemical transformation Which bonds are broken and in what order Which bonds are formed and in what order What is the relative rate of each step A complete mechanism accounts for all reactants consumed and all products formed
Reaction Mechanism ▪ An overall description of how a reaction occurs at each stage of a chemical transformation ▪ Which bonds are broken and in what order ▪ Which bonds are formed and in what order ▪ What is the relative rate of each step ▪ A complete mechanism accounts for all reactants consumed and all products formed 6-2 How Organic Reactions Occur: Mechanisms

How Organic Reactions Occur:Mechanisms All chemical reactions involve bond breaking and bond making Two ways a covalent two-electron bond can break: 1.Symmetrical or Homolytic One electron remains A·+B with each product Half-headed arrow,“fishhook”, fragment indicates movement of one 2.Unsymmetrical or Heterolytic electron Both bonding electrons remain with one A:日 A++:B product fragment, Full-headed arrow indicates leaving the other with movement of two electrons a vacant orbital
All chemical reactions involve bond breaking and bond making Two ways a covalent two-electron bond can break: 1. Symmetrical or Homolytic ▪ One electron remains with each product fragment 2. Unsymmetrical or Heterolytic ▪ Both bonding electrons remain with one product fragment, leaving the other with a vacant orbital Half-headed arrow, “fishhook”, indicates movement of one electron Full-headed arrow indicates movement of two electrons How Organic Reactions Occur: Mechanisms
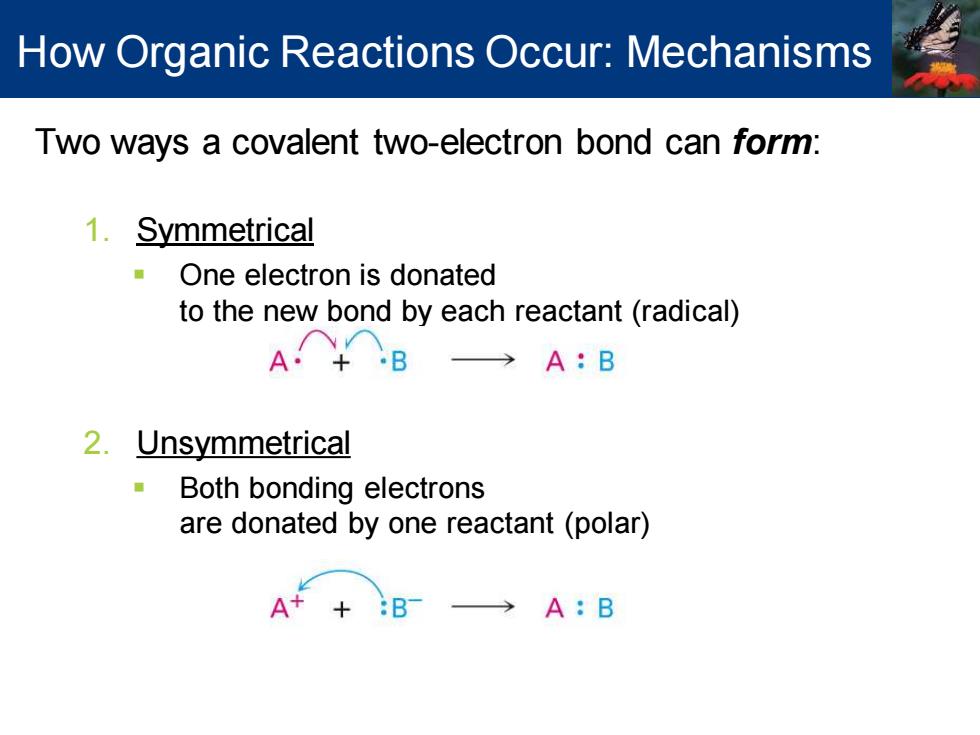
How Organic Reactions Occur:Mechanisms Two ways a covalent two-electron bond can form: 1.Symmetrical One electron is donated to the new bond by each reactant(radical) →A:B 2.Unsymmetrical Both bonding electrons are donated by one reactant(polar) A++ :BT A:B
Two ways a covalent two-electron bond can form: 1. Symmetrical ▪ One electron is donated to the new bond by each reactant (radical) 2. Unsymmetrical ▪ Both bonding electrons are donated by one reactant (polar) How Organic Reactions Occur: Mechanisms
How Organic Reactions Occur:Mechanisms Radical reaction Process that involves symmetrical bond breaking and bond making Radical (free radical) A neutral chemical species that contains an odd number of electrons and has a single,unpaired electron in one of its orbitals Polar reaction Process that involves unsymmetrical bond breaking and bond making Involve species that have an even number of electrons (have only electron pairs in their orbitals) Common in both organic and biological chemistry
Radical reaction ▪ Process that involves symmetrical bond breaking and bond making ▪ Radical (free radical) ▪ A neutral chemical species that contains an odd number of electrons and has a single, unpaired electron in one of its orbitals Polar reaction ▪ Process that involves unsymmetrical bond breaking and bond making ▪ Involve species that have an even number of electrons (have only electron pairs in their orbitals) ▪ Common in both organic and biological chemistry How Organic Reactions Occur: Mechanisms