
Radical Reactions Termination Two radicals combine to end the chain reaction No new radical species is formed :d○+○ci: :CI:CI: :c.○+cH3 → :CI:CH3 Possible termination steps Hec○+cH → H3C:CH3
Termination • Two radicals combine to end the chain reaction • No new radical species is formed Radical Reactions
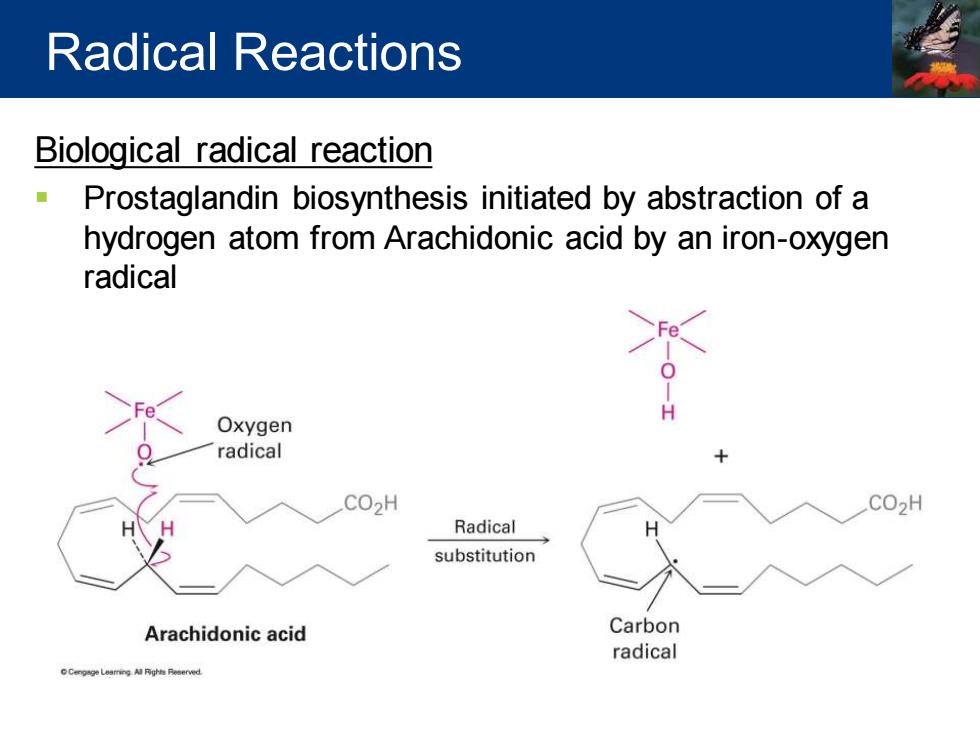
Radical Reactions Biological radical reaction Prostaglandin biosynthesis initiated by abstraction of a hydrogen atom from Arachidonic acid by an iron-oxygen radical Oxygen radical CO2H CO2H Radical substitution Arachidonic acid Carbon radical CCg学Leaming Al Pigh Peserved
Biological radical reaction ▪ Prostaglandin biosynthesis initiated by abstraction of a hydrogen atom from Arachidonic acid by an iron-oxygen radical Radical Reactions
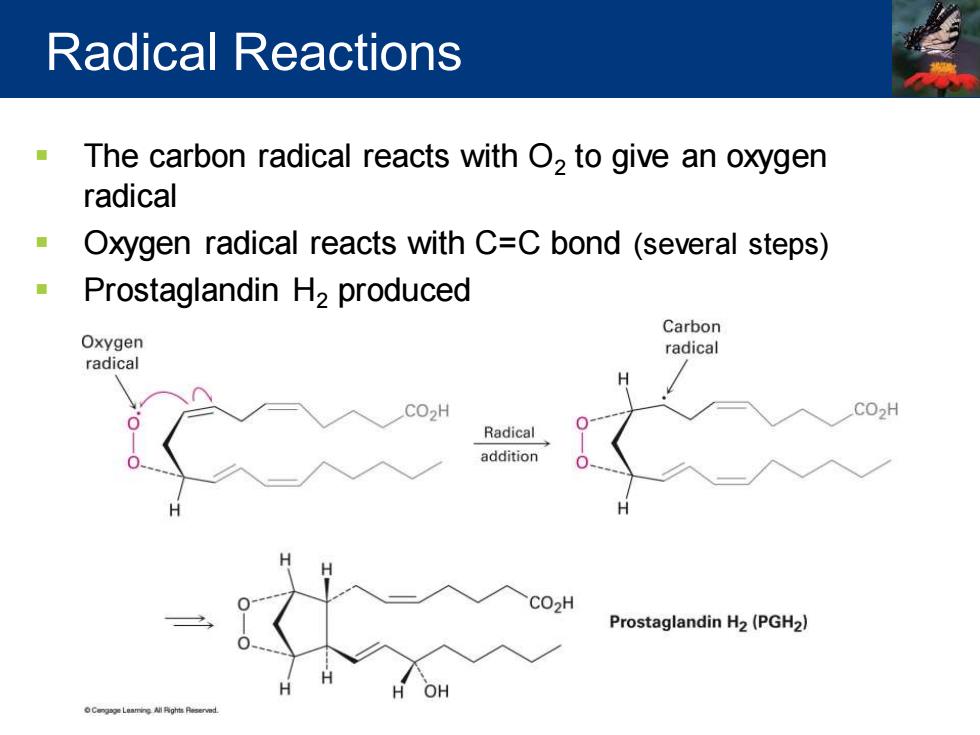
Radical Reactions The carbon radical reacts with O2 to give an oxygen radical Oxygen radical reacts with C=C bond (several steps) Prostaglandin H2 produced Carbon Oxygen radical radical H CO2H CO2H Radical addition CO2H Prostaglandin H2(PGH2) OH Cengge Leamine All Pights Pesernd
▪ The carbon radical reacts with O2 to give an oxygen radical ▪ Oxygen radical reacts with C=C bond (several steps) ▪ Prostaglandin H2 produced Radical Reactions

6-4 Polar Reactions Polar reactions Occur because of electrical attraction between positively polarized and negatively polarized centers on functional groups in molecules Bond polarity Most organic compounds are electrically neutral,they have no net charge Certain bonds within a molecule are polar Consequence of an unsymmetrical electron distribution in a bond 。 Due to the difference in electronegativity of the bonded atoms
Polar reactions • Occur because of electrical attraction between positively polarized and negatively polarized centers on functional groups in molecules Bond polarity • Most organic compounds are electrically neutral, they have no net charge • Certain bonds within a molecule are polar • Consequence of an unsymmetrical electron distribution in a bond • Due to the difference in electronegativity of the bonded atoms. 6-4 Polar Reactions

Polar Reactions Certain bonds within molecules,particularly those in functional groups,are polar Oxygen,nitrogen,fluorine,and chlorine are more electronegative than carbon Carbon is always positively polarized (8+)when bonded to more electronegative elements ( Carbon is negatively polarized (-)when bonded to metals 6- H H H H Chloromethane Methyllithium eLaming.An Pigtes Rooerved
Certain bonds within molecules, particularly those in functional groups, are polar ▪ Oxygen, nitrogen, fluorine, and chlorine are more electronegative than carbon ▪ Carbon is always positively polarized (d+) when bonded to more electronegative elements ▪ Carbon is negatively polarized (d– ) when bonded to metals Polar Reactions