
Consider the water molecule (non-linear,3 atoms->3 fundamental vibrational modes) Figure 12-2 These are only the Fundamental vibrational modes,these can combine and multiply to give rise to numerous other modes of vibration. These more complicated modes give rise to the numerous absorptions in ar IR spectra,and since different molecules will have different IR spectra,we can use IR to help identify molecules. The most complex(and informative)region is called the fingerprint region (600-1400cm),and this contains the wagging,twisting,scissoring and rocking vibrations. The region that contains the simple stretching vibrations is typically from 3500 to 1600cm,and this is the area we will focus our studies on. IR Active and IR Inactive Vibrations Not all molecular vibrations absorb IR radiation. To understand which ones do(and which do not)absorb we need to consider how an electromagnetic field interacts with the chemical bond. Important points are: (1)A chemical bond which is polarized has a dipole moment(i.e.charge separation). (2)An electric field will interact with the polar bond,causing it either to stretch or compress. (3)An electromagnetic wave has a rapidly reversing electric field,so this radiation rapidly compresses and stretches the polar bond. (4)If the frequency of the stretching and compression is at the frequency of the molecule's natural rate of vibration,then energy may be absorbed. Vibrations of bonds with dipole moments normally result in the absorption of IR radiation,and are said to be IR active. Ch12 IR and MS Page6
Consider the water molecule (non-linear, 3 atoms → 3 fundamental vibrational modes). Figure 12-2 These are only the Fundamental vibrational modes, these can combine and multiply to give rise to numerous other modes of vibration. These more complicated modes give rise to the numerous absorptions in an IR spectra, and since different molecules will have different IR spectra, we can use IR to help identify molecules. The most complex (and informative) region is called the fingerprint region (600-1400cm-1) , and this contains the wagging, twisting, scissoring and rocking vibrations. The region that contains the simple stretching vibrations is typically from 3500 to 1600cm-1, and this is the area we will focus our studies on. IR Active and IR Inactive Vibrations Not all molecular vibrations absorb IR radiation. To understand which ones do (and which do not) absorb we need to consider how an electromagnetic field interacts with the chemical bond. Important points are: (1) A chemical bond which is polarized has a dipole moment (i.e. charge separation). (2) An electric field will interact with the polar bond, causing it either to stretch or compress. (3) An electromagnetic wave has a rapidly reversing electric field, so this radiation rapidly compresses and stretches the polar bond. (4) If the frequency of the stretching and compression is at the frequency of the molecule's natural rate of vibration, then energy may be absorbed. Vibrations of bonds with dipole moments normally result in the absorption of IR radiation, and are said to be IR active. Ch12 IR and MS Page6
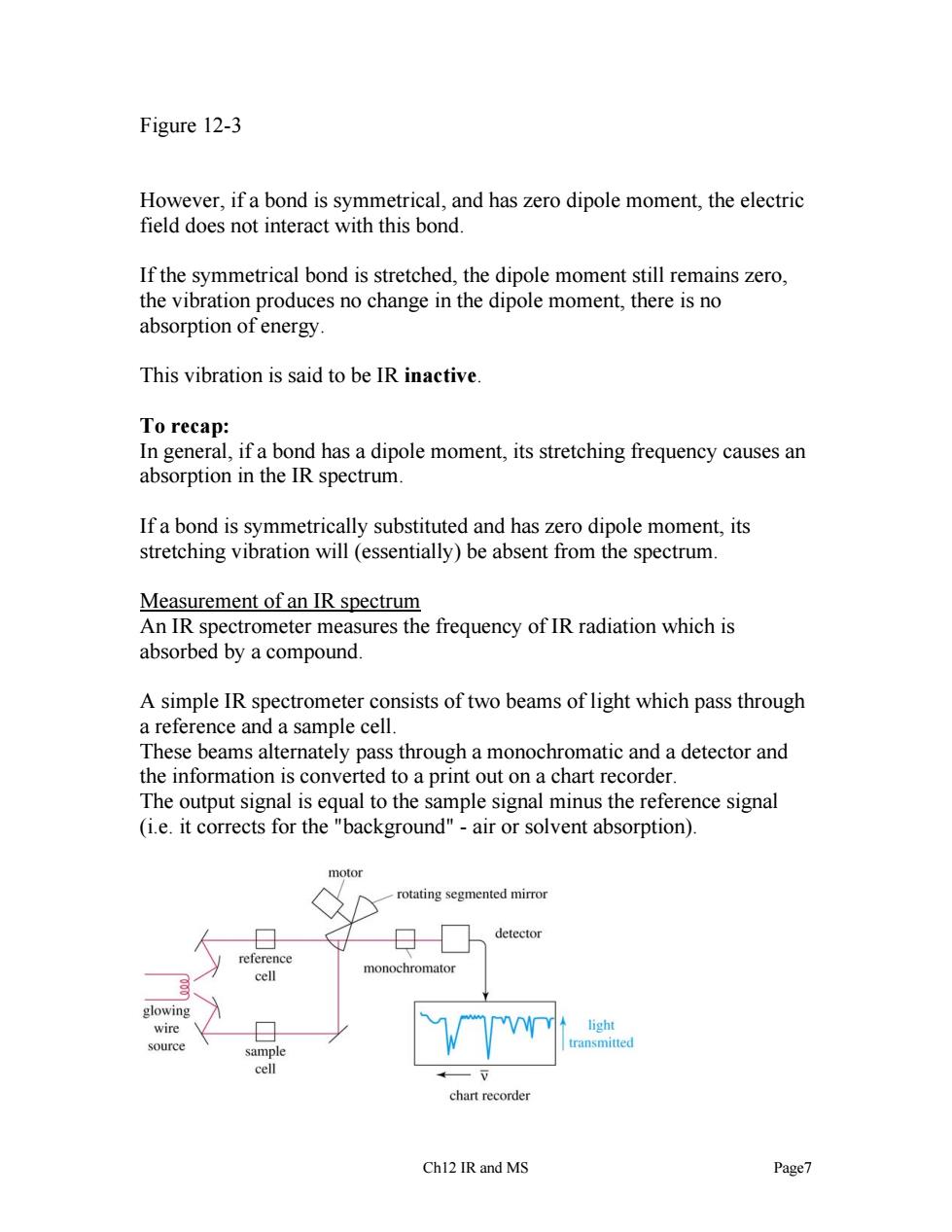
Figure 12-3 However,if a bond is symmetrical,and has zero dipole moment,the electric field does not interact with this bond. If the symmetrical bond is stretched,the dipole moment still remains zero. the vibration produces no change in the dipole moment,there is no absorption of energy. This vibration is said to be IR inactive. To recap: In general,ifa bond has a dipole moment,its stretching frequency causes an absorption in the IR spectrum. If a bond is symmetrically substituted and has zero dipole moment,its stretching vibration will (essentially)be absent from the spectrum. Measurement of an IR spectrum An IR spectrometer measures the frequency of IR radiation which is absorbed by a compound. A simple IR spectrometer consists of two beams of light which pass through a reference and a sample cell. These beams alternately pass through a monochromatic and a detector and the information is converted to a print out on a chart recorder The output signal is equal to the sample signal minus the reference signal (i.e.it corrects for the "background"-air or solvent absorption). rotating segmented miro monochromato chart recorder Chl2 IR and MS Page7
Figure 12-3 However, if a bond is symmetrical, and has zero dipole moment, the electric field does not interact with this bond. If the symmetrical bond is stretched, the dipole moment still remains zero, the vibration produces no change in the dipole moment, there is no absorption of energy. This vibration is said to be IR inactive. To recap: In general, if a bond has a dipole moment, its stretching frequency causes an absorption in the IR spectrum. If a bond is symmetrically substituted and has zero dipole moment, its stretching vibration will (essentially) be absent from the spectrum. Measurement of an IR spectrum An IR spectrometer measures the frequency of IR radiation which is absorbed by a compound. A simple IR spectrometer consists of two beams of light which pass through a reference and a sample cell. These beams alternately pass through a monochromatic and a detector and the information is converted to a print out on a chart recorder. The output signal is equal to the sample signal minus the reference signal (i.e. it corrects for the "background" - air or solvent absorption). Ch12 IR and MS Page7