
有机化学oRcAn1©©ⅫEMISTRY 孟饼:王依儒87092829l 理学院爱化素理科接2层206 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 4 Structure and Stereochemistry of Alkanes Key Notes Mechanisms;Conformation;Ring strain; Conformers;Cis-trans isomers;
Chapter 4 Structure and Stereochemistry of Alkanes Key Notes Mechanisms; Conformation; Ring strain; Conformers; Cis-trans isomers; Organic Chemistry, 6th Edition L. G. Wade, Jr. 有机化学ORGANIC CHEMISTRY 主讲:王俊儒 87092829(o) 理学院应化系理科楼2层C206
自秋转对 CONTENTS 教华税 Classification of Hydrocarbons ■Physical properties Reactions Mechanisms of Alkanes Structure Conformation of Alkanes Conformation of Cycloalkanes Homework: 4-7140:4-16;:4-18(a,b,d):P147)
CONTENTS ◼Classification of Hydrocarbons ◼Physical properties ◼Reactions & Mechanisms of Alkanes ◼Structure & Conformation of Alkanes ◼Conformation of Cycloalkanes Homework: 4-7(P140); 4-16; 4-18(a,b,d);(P147))
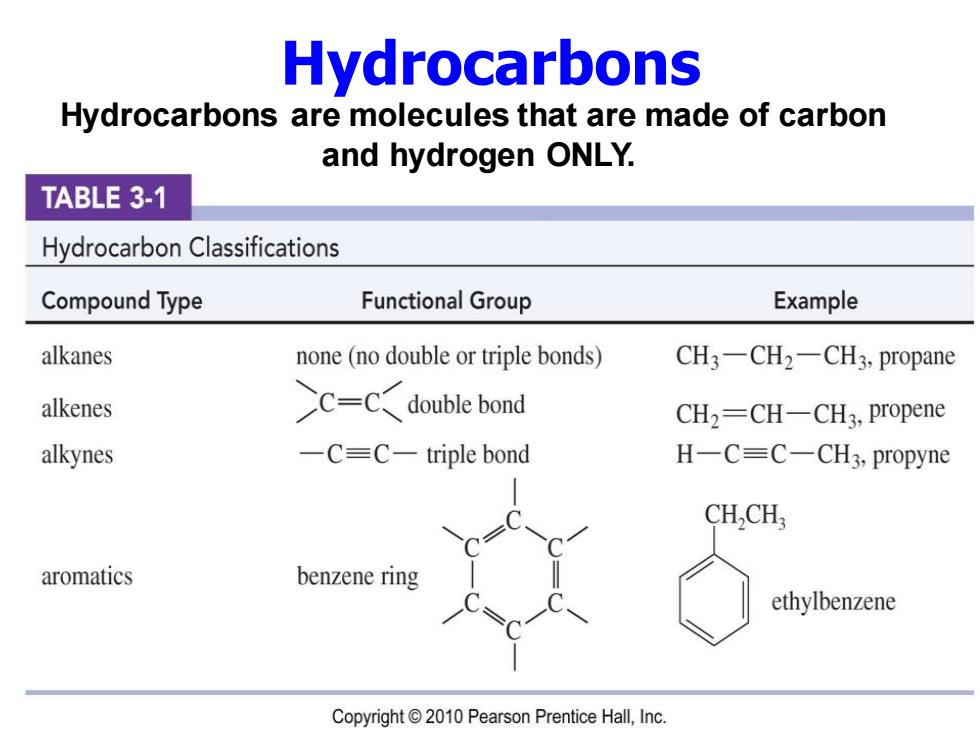
Hydrocarbons Hydrocarbons are molecules that are made of carbon and hydrogen ONLY. TABLE 3-1 Hydrocarbon Classifications Compound Type Functional Group Example alkanes none(no double or triple bonds) CH3-CH2-CH3,propane alkenes C-Cdouble bond CH2=CH-CH3,propene alkynes -C=C-triple bond H一C=C-CH3,propyne CH,CH, aromatics benzene ring ethylbenzene Copyright 2010 Pearson Prentice Hall,Inc
Hydrocarbons are molecules that are made of carbon and hydrogen ONLY. Hydrocarbons
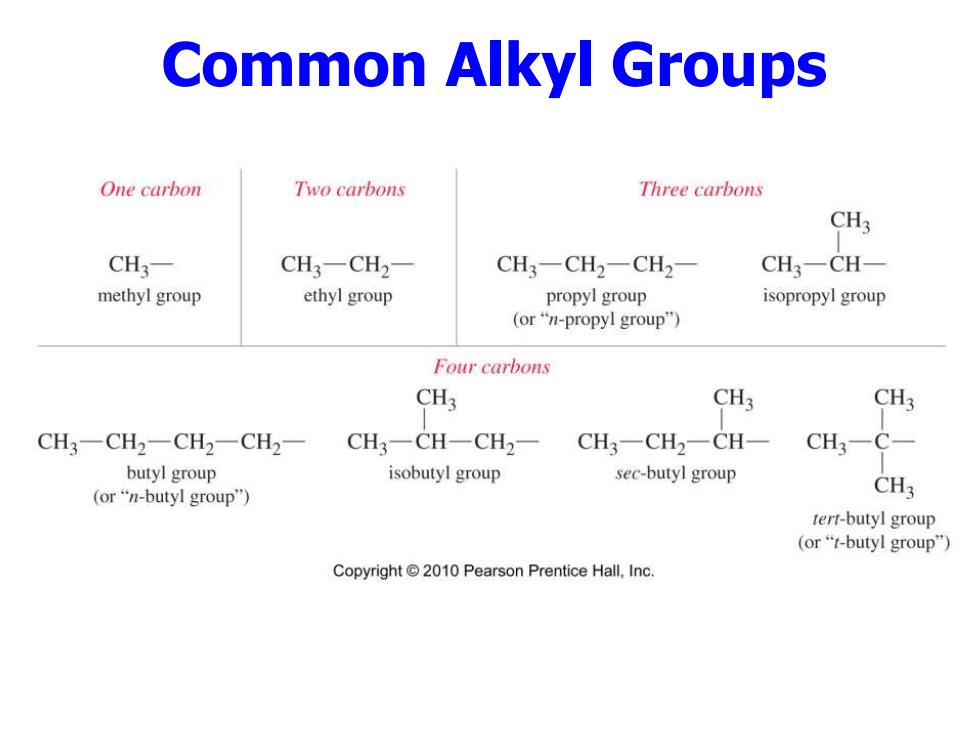
Common Alkyl Groups One carbon Two carbons Three carbons CH3 CH3- CH3一CH2 CH3一CH2一CH2 CH3-CH- methyl group ethyl group propyl group isopropyl group (or"n-propyl group") Four carbons CH3 CH3 CH3 CH3-CH2一CH2-CH2- CH3-CH-CH2- CH3-CH2-CH- CH3-C- butyl group isobutyl group sec-butyl group (or"n-butyl group") CH3 tert-butyl group (or“t-butyl group") Copyright 2010 Pearson Prentice Hall,Inc
Common Alkyl Groups
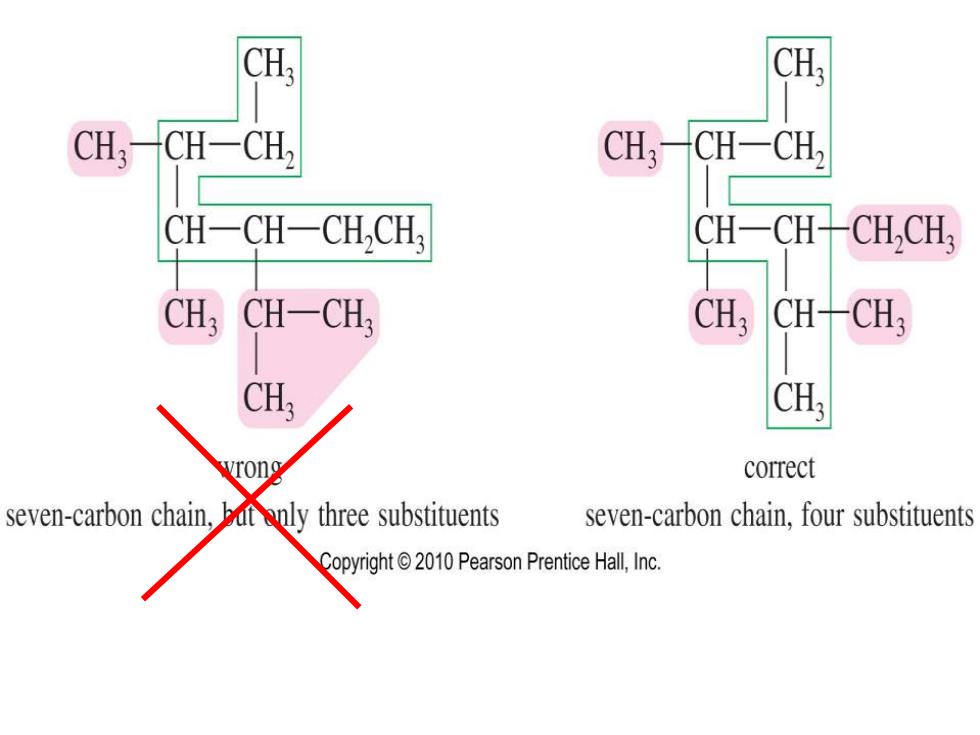
CH: CH,CH-CH, CH,一CH-CH CH-CH-CH,CH; CH一CHCH,CH, CH3 CH-CH: CH, CH-CH3 CH: CH: correct seven-carbon chain,but only three substituents seven-carbon chain,four substituents Copyright2010 Pearson Prentice Hall,Inc