有机化学ORGANIC CHEMISTRY 主讲:主依儒87092829(0) 理学院应化系理科接2层C206 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds Key Notes Enol-keto tautomerism,Todoform Reaction,Aldol Condensation,Claisen Condensation,Acetoacetic Ester Synthesis; The Michael Reactionj Homework:P806:23-28,23-30(a,b,c,d)
Chapter 23 Condensation and Alpha Substitution of Carbonyl Compounds Organic Chemistry, 6th Edition L. G. Wade, Jr. Key Notes Enol–keto tautomerism,Iodoform Reaction,Aldol Condensation,Claisen Condensation,Acetoacetic Ester Synthesis; The Michael Reaction; 有机化学ORGANIC CHEMISTRY 主讲:王俊儒 87092829(O) 理学院应化系理科楼2层C206 Homework: P806: 23-28; 23-30(a,b,c,d)

自秋标 CONTENTS 1.REVIEW: Enol -keto tautomerism 2.Iodoform and Haloform Reaction(碘仿反应) 3.Aldol Condensation(羟醛缩合) 4.Claisen Condensation(酯缩合) 5.Acetoacetic Ester Synthesis;Malonic Ester Synthesis(三乙合成;丙二酸酯合成路线) 6.Conjugate Additions: The Michael Reaction(迈克尔加成)
CONTENTS 1. REVIEW: Enol – keto tautomerism 2. Iodoform and Haloform Reaction(碘仿反应) 3. Aldol Condensation(羟醛缩合) 4. Claisen Condensation (酯缩合) 5. Acetoacetic Ester Synthesis;Malonic Ester Synthesis(三乙合成;丙二酸酯合成路线) 6. Conjugate Additions: The Michael Reaction(迈克尔加成)
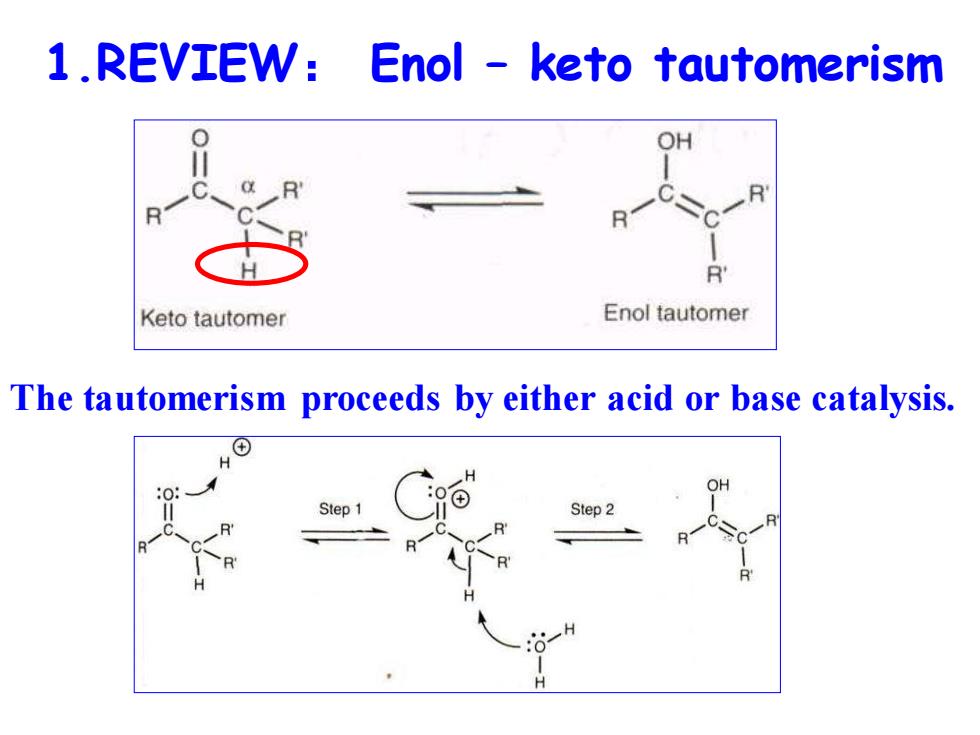
1.REVIEW: Enol keto tautomerism OH R Keto tautomer Enol tautomer The tautomerism proceeds by either acid or base catalysis. Step 1 Step 2 H
1.REVIEW: Enol – keto tautomerism The tautomerism proceeds by either acid or base catalysis
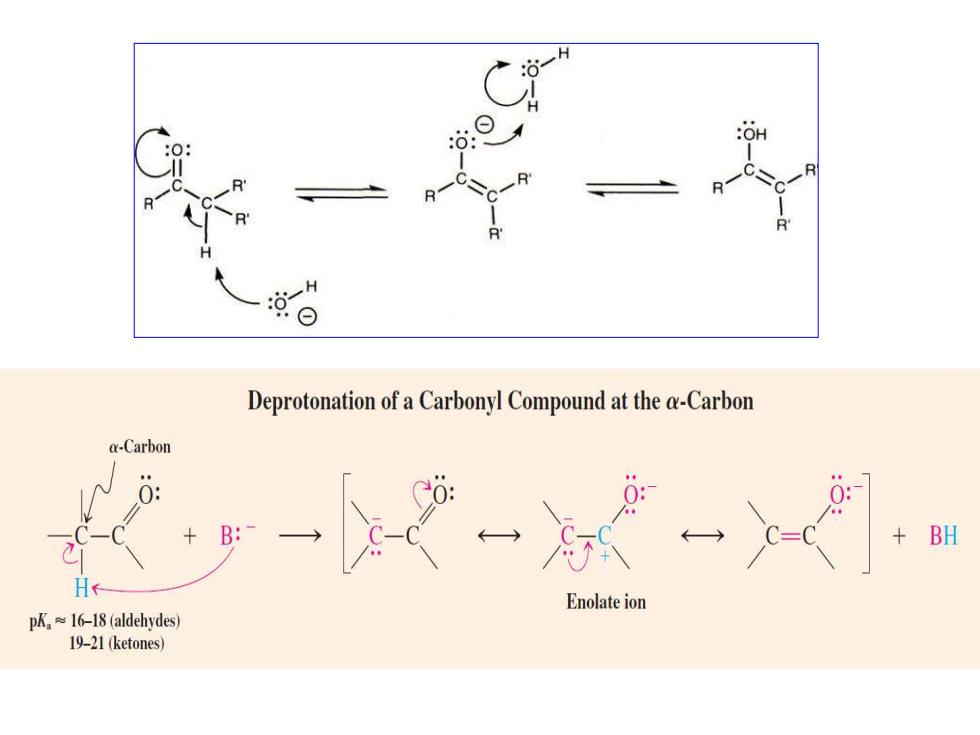
:OH H Deprotonation of a Carbonyl Compound at the a-Carbon a-Carbon 0: BH Enolate ion pk≈l6-l8(aldehydes) 19-21(ketones)
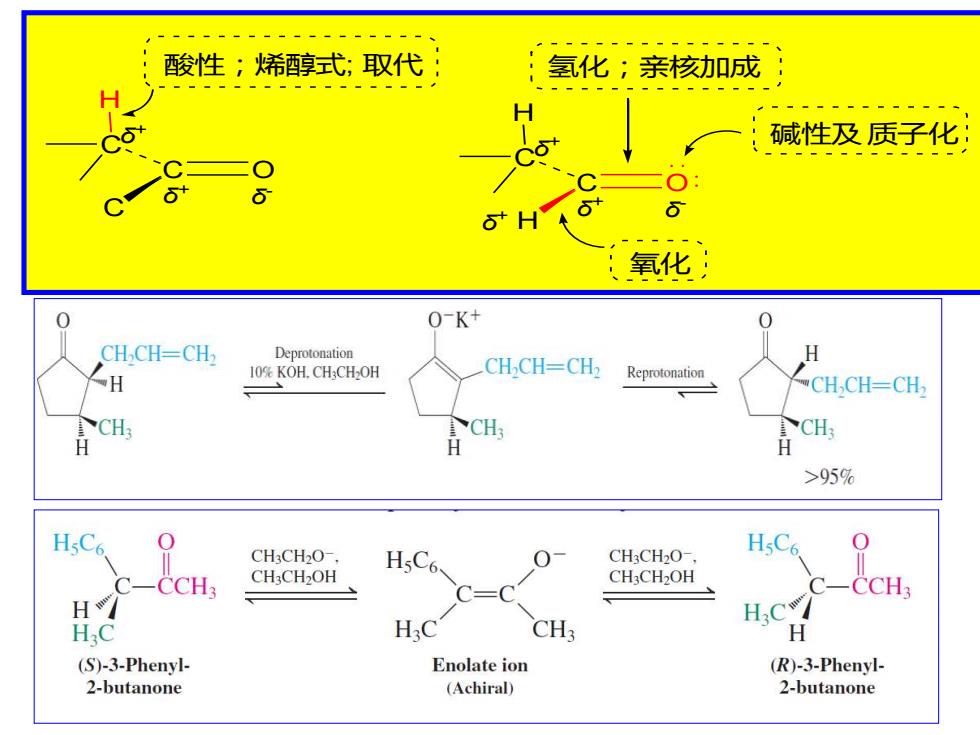
酸性;烯醇式;取代 氢化;亲核加成: 碱性及质子化: ● 6 :氧化 O-K+ 0 CH,CH-CH2 Deprotonation H 10%KOH.CHCH-OH CHCH=CH2 H Reprotonation CH,CH-CH CH. CH H H >95% HsC6 CH3CH2O-. HsC6 CH3CH2O-. HsC6 -CCH: CH3CH2OH CH3CH2OH CCH H.C* HC H.C CH3 H (S)-3-Phenyl- Enolate ion (R)-3-Phenyl- 2-butanone (Achiral) 2-butanone
C O C C δ - δ + H δ + C O H C δ - δ + H δ + 酸性;烯醇式; 取代 氢化;亲核加成 碱性及 质子化 氧化 δ +