自秋不转大对 教华税 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 21 Carboxylic Acids http://www.study-organic-chemistry.com/ By Junru Wang Email:wangjr07@163.com
By Junru Wang Email: wangjr07@163.com Chapter 21 Carboxylic Acids Organic Chemistry, 6th Edition L. G. Wade, Jr. http://www.study-organic-chemistry.com/

CONTENT ■REVIEW:NAMING STRUCTURE AND PROPERTIES NUCLEOPHILIC SUBSTITUTION ■PREPARATIONS ■REACTIONS ■ENOLATE REACTIONS
CONTENT ◼REVIEW:NAMING ◼STRUCTURE AND PROPERTIES ◼NUCLEOPHILIC SUBSTITUTION ◼PREPARATIONS ◼REACTIONS ◼ENOLATE REACTIONS

Sec 1 STRUCTURE AND PROPERTIES Carbon is sp2 hybridized. 1.32A Bond angles are close to 124/2s 1.23A 120°. )106 1.10A 0.97A H O-H eclipsed with C=O,to H 11100 H get overlap of zorbital bond angles bond lengths with orbital of lone pair or. oxygen. Resonance Stabilization R-C-0-H+H,0: R- +H,0t pK=5 (K≡105) acid carboxylate
◼Carbon is sp2 hybridized. ◼Bond angles are close to 120 . ◼O-H eclipsed with C=O, to get overlap of orbital with orbital of lone pair on oxygen. ◼Resonance Stabilization Sec 1 STRUCTURE AND PROPERTIES
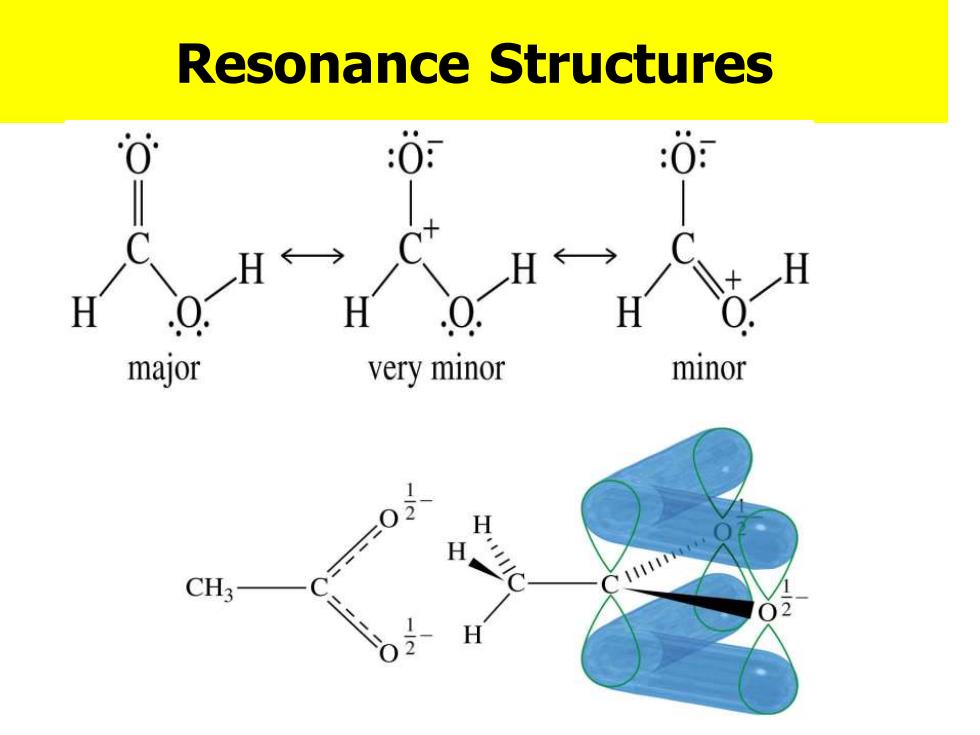
Resonance Structures :0: :0 H←→ H H H H H major very minor minor H CH3
Resonance Structures
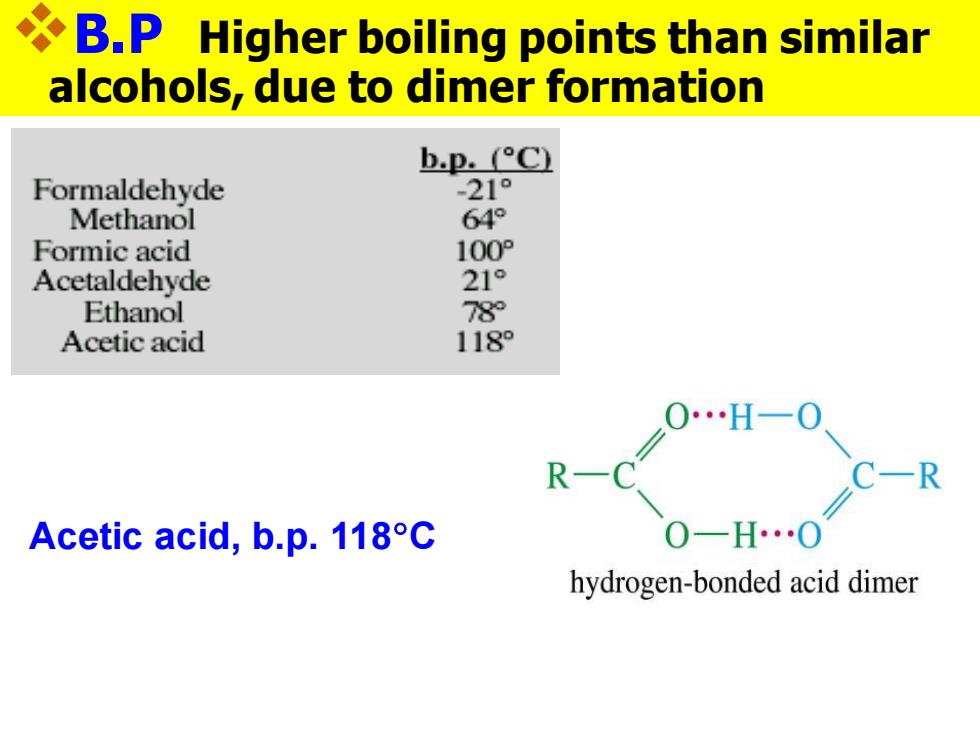
B.P Higher boiling points than similar alcohols,due to dimer formation b.p.°C Formaldehyde -21° Methanol 64° Formic acid 100P Acetaldehyde 21° Ethanol 78 Acetic acid 118 0H一0 R- Acetic acid,b.p.118C hydrogen-bonded acid dimer
❖B.P Higher boiling points than similar alcohols, due to dimer formation Acetic acid, b.p. 118C