Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 19 Aldehydes and Ketones http://www.study-organic-chemistry.com/
Chapter 19 Aldehydes and Ketones Organic Chemistry, 6th Edition L. G. Wade, Jr. http://www.study-organic-chemistry.com/
自秋转大好 Content Carbonyl Structure Properties Preparation >Functional group transformations >Carbon-carbon bond formation >C-C Bond cleavage Reactions of Aldehydes and Ketones ●C=O ●o-H,a-C
Content ◼Carbonyl Structure & Properties ◼Preparation ➢Functional group transformations ➢Carbon-carbon bond formation ➢C-C Bond cleavage ◼Reactions of Aldehydes and Ketones ⚫C=O ⚫-H, - C
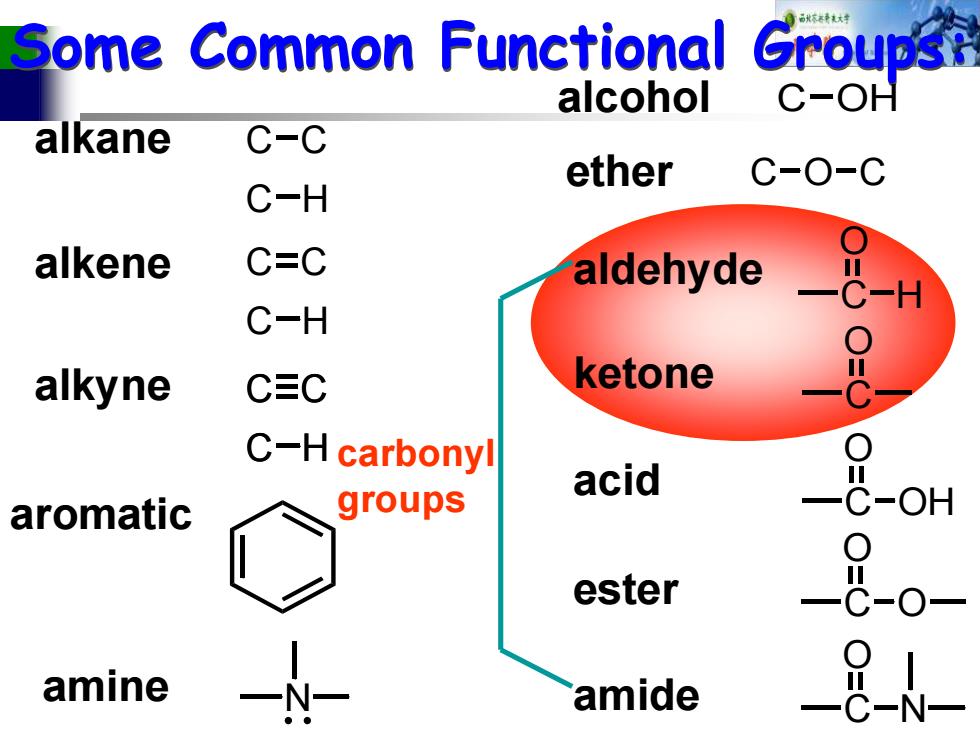
Some Common Functional Groupsi alcohol C-OH alkane C-C ether C-O-C C-H alkene C=C aldehyde C-H alkyne C三C ketone C-H carbonyl acid aromatic groups OH ester amine amide N
Some Common Functional Groups: C C C H alkane alkene C C C H alkyne C C C H C C C H aromatic alcohol C OH ether C O C C O H aldehyde C O ketone C O OH acid C O ester O C O N amide N amine carbonyl groups

Carbonyl Compounds TABLE 18-1 Some Common Classes of Carbonyl Compounds Class General Formula Class General Formula ketones R-C-R aldehydes carboxylic acids OH acid chlorides esters R -O-R amides Copyright 2005 Pearson Prentice Hall,Inc
Carbonyl Compounds
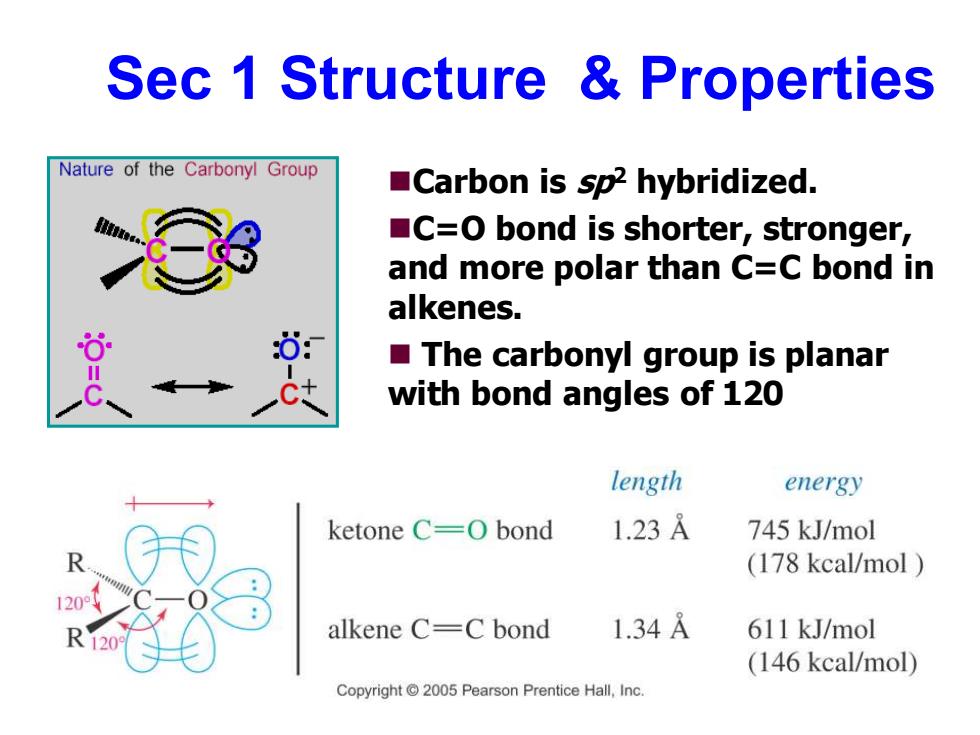
Sec 1 Structure Properties Nature of the Carbonyl Group Carbon is sp2 hybridized. C=O bond is shorter,stronger, and more polar than C=C bond in alkenes. The carbonyl group is planar with bond angles of 120 length energy ketone C=O bond 1.23A 745 kJ/mol (178 kcal/mol alkene C=C bond 1.34A 611 kJ/mol (146 kcal/mol) Copyright 2005 Pearson Prentice Hall,Inc
Sec 1 Structure & Properties ◼Carbon is sp2 hybridized. ◼C=O bond is shorter, stronger, and more polar than C=C bond in alkenes. ◼ The carbonyl group is planar with bond angles of 120