Organic Chemistry,6th Edition L.G.Wade,Jr Chapter 6 Stereochemistry
Chapter 6 Stereochemistry Organic Chemistry, 6th Edition L. G. Wade, Jr

Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 6 Stereochemistry Key Notes Chiralenantiomers;Chirality Chiral center;Optical rotations;R/S,D/L;Diastereomers;mesoforms; Resolution of enantiomers;
Chapter 6 Stereochemistry Organic Chemistry, 6th Edition L. G. Wade, Jr. Key Notes Chiral enantiomers; Chirality & Chiral center; Optical rotations; R /S ,D/L; Diastereomers ; meso forms; Resolution of enantiomers;
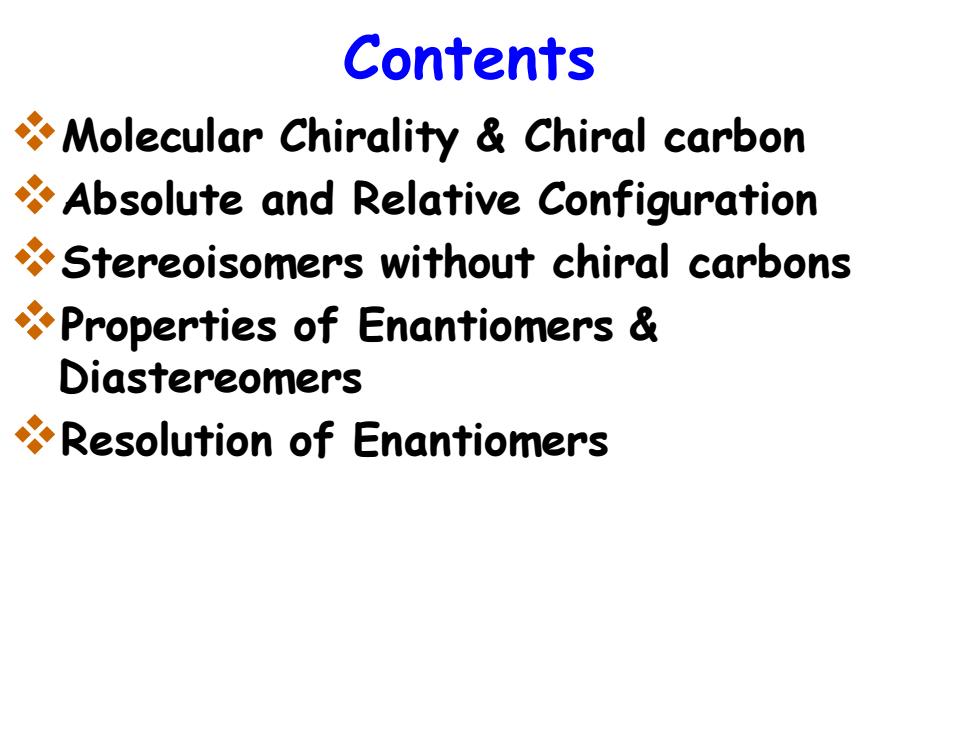
Contents Molecular Chirality Chiral carbon Absolute and Relative Configuration Stereoisomers without chiral carbons Properties of Enantiomers Diastereomers Resolution of Enantiomers
Contents ❖Molecular Chirality & Chiral carbon ❖Absolute and Relative Configuration ❖Stereoisomers without chiral carbons ❖Properties of Enantiomers & Diastereomers ❖Resolution of Enantiomers

Biological Significance of Chirality Since most of the natural (biological)environment consists of enantiomeric molecules (amino acids, nucleosides,carbohydrates and phospholipids are chiral molecules),then enantiomers will display different properties.Then,in our body: Enantiomers Drug Enzyme Tight Binding Weak Binding
◆Biological Significance of Chirality
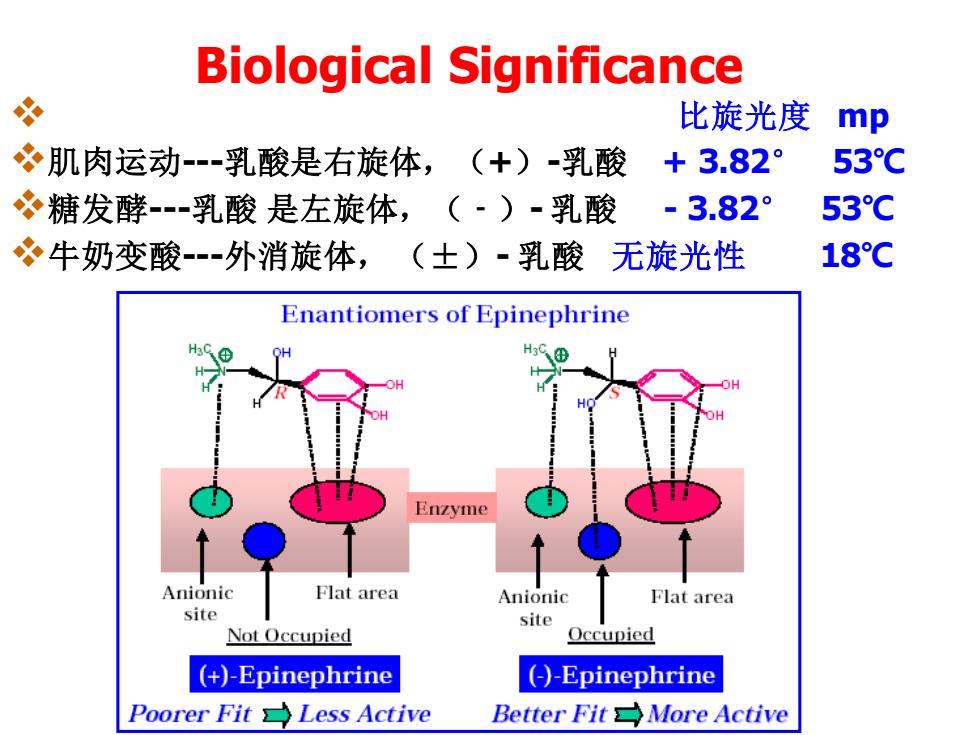
Biological Significance 必 比旋光度 mp 肌肉运动--乳酸是右旋体,(+)-乳酸+3.82° 53°C 糖发酵乳酸是左旋体,(·)-乳酸 -3.82° 53C 牛奶变酸外消旋体,(士)·乳酸 无旋光性 18°C Enantiomers of Epinephrine H3C⊕ Enzyme Anionic Flat area Anionic Flat area site site Not Occupied Occupied (+)-Epinephrine (-)-Epinephrine Poorer Fit Less Active Better Fit More Active
Biological Significance ❖ 比旋光度 mp ❖肌肉运动---乳酸是右旋体,(+)-乳酸 + 3.82° 53℃ ❖糖发酵---乳酸 是左旋体,(﹣)- 乳酸 - 3.82° 53℃ ❖牛奶变酸---外消旋体, (±)- 乳酸 无旋光性 18℃