
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 08 09 Alkenes By Junru Wang Email:wangjr07@163.com 西北农林科技大学理学院
西北农林科技大学理学院 By Junru Wang Email: wangjr07@163.com Chapter 08 & 09 Alkenes Organic Chemistry, 6th Edition L. G. Wade, Jr

Content Introduction Properties Preparation Reactions of alkenes Electrophilic addition -Carbocation stabilization -Free-Radical Addition of HBr -Reduction and oxidation Conjugated dienes
Content Introduction & Properties Preparation Reactions of alkenes Electrophilic addition Carbocation stabilization Free-Radical Addition of HBr Reduction and oxidation Conjugated dienes

Key Notes Electrophile Electrophilic addition Carbocation stability Conjugated dienes
Key Notes Electrophile Electrophilic addition Carbocation stability Conjugated dienes

Sec⊥Introduction& Properties Hydrocarbon with carbon-carbon double bonds Sometimes called olefins,"oil-forming gas" Pi bond is the functional group. More reactive than sigma bond. Bond dissociation energies: C=C BDE 611 kJ/mol C-C BDE -347 kJ/mol -Pi bond 264 kJ/mol
Sec 1 Introduction & Properties Hydrocarbon with carbon-carbon double bonds Sometimes called olefins, “oil-forming gas ” Pi bond is the functional group. More reactive than sigma bond. Bond dissociation energies: C=C BDE 611 kJ/mol C-C BDE -347 kJ/mol Pi bond 264 kJ/mol
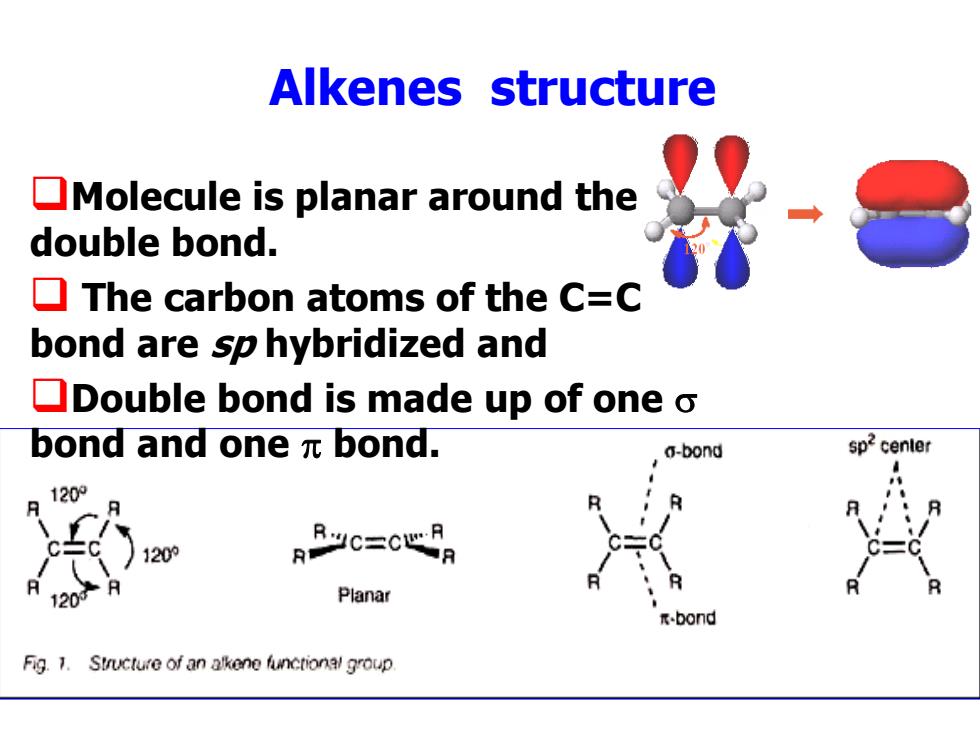
Alkenes structure Molecule is planar around the double bond. The carbon atoms of the C=C bond are sp hybridized and Double bond is made up of one o bond and one元bond. a-bond sp2 center 1209 120的 RRc=c心RR 120 Planar -bond Fig.1.Structure of an alkene functional group
Alkenes structure Molecule is planar around the double bond. The carbon atoms of the C=C bond are sp hybridized and Double bond is made up of one bond and one bond