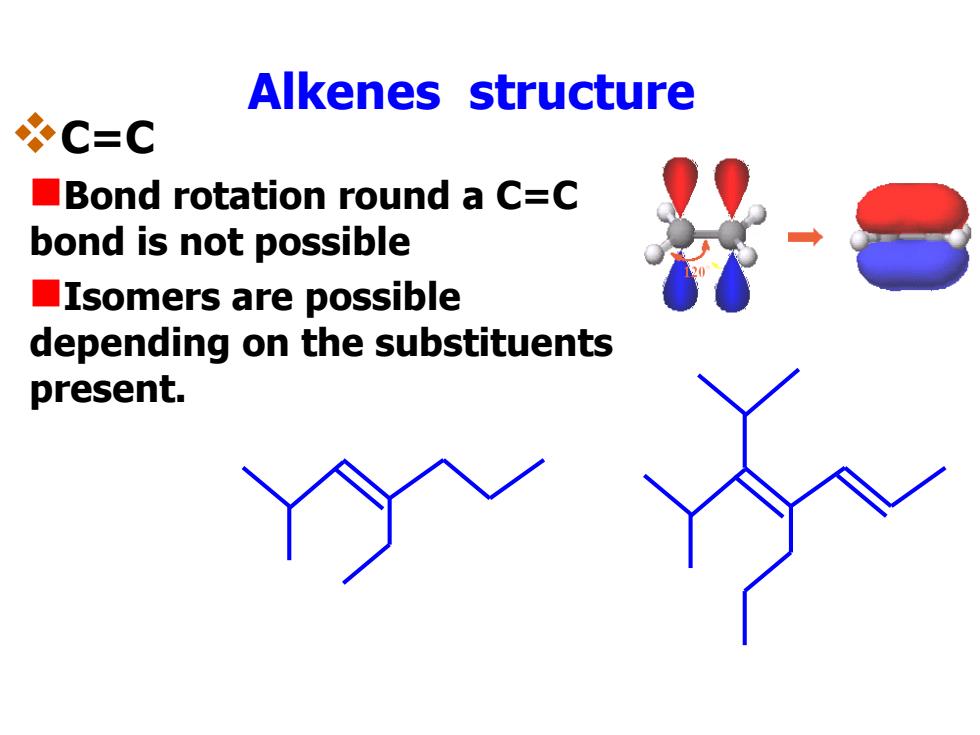
Alkenes structure C=C Bond rotation round a C=C bond is not possible ■Isomers are possible depending on the substituents present
Alkenes structure C=C Bond rotation round a C=C bond is not possible Isomers are possible depending on the substituents present
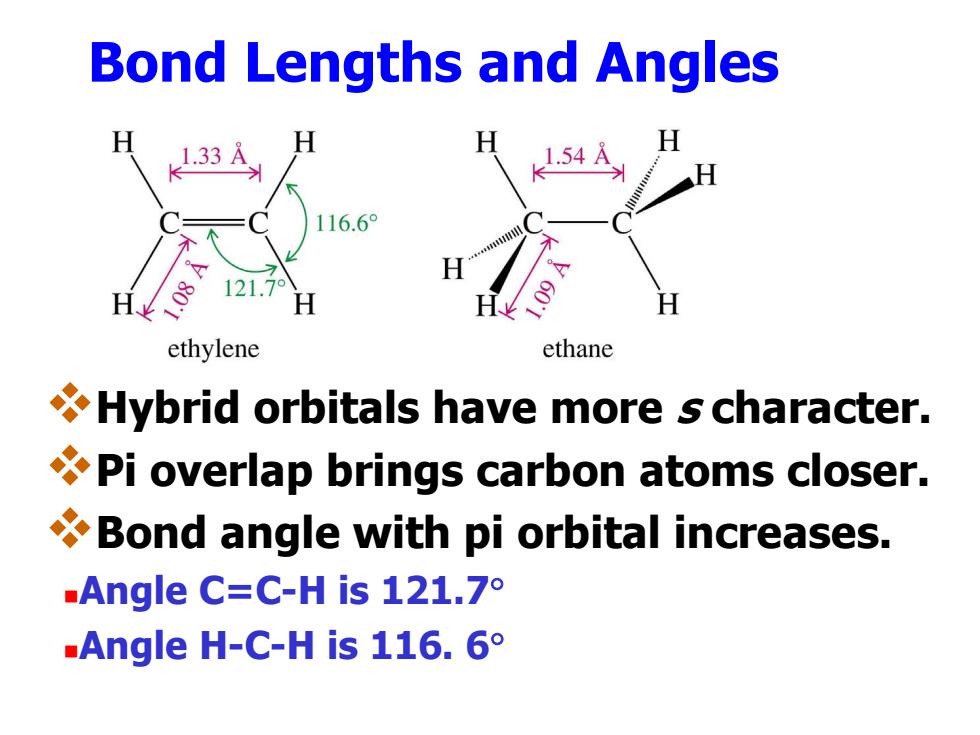
Bond Lengths and Angles 1.33 116.6° 121.7° ethylene ethane Hybrid orbitals have more s character. Pi overlap brings carbon atoms closer. Bond angle with pi orbital increases. .Angle C=C-His121.7° Angle H-C-His116.6°
Bond Lengths and Angles Hybrid orbitals have more s character. Pi overlap brings carbon atoms closer. Bond angle with pi orbital increases. Angle C=C-H is 121.7 Angle H-C-H is 116. 6
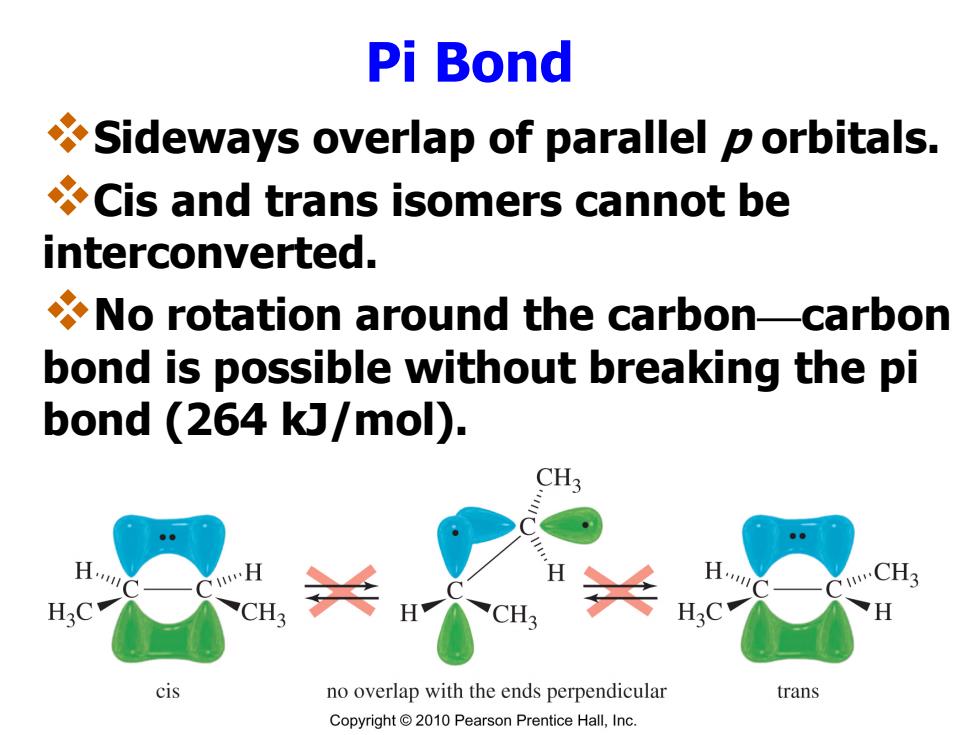
Pi Bond Sideways overlap of parallel p orbitals. Cis and trans isomers cannot be interconverted. No rotation around the carbon-carbon bond is possible without breaking the pi bond (264 kJ/mol). CH3 B. CH3 H.C CH3 H.C cis no overlap with the ends perpendicular trans Copyright2010 Pearson Prentice Hall,Inc
Pi Bond Sideways overlap of parallel p orbitals. Cis and trans isomers cannot be interconverted. No rotation around the carbon—carbon bond is possible without breaking the pi bond (264 kJ/mol)
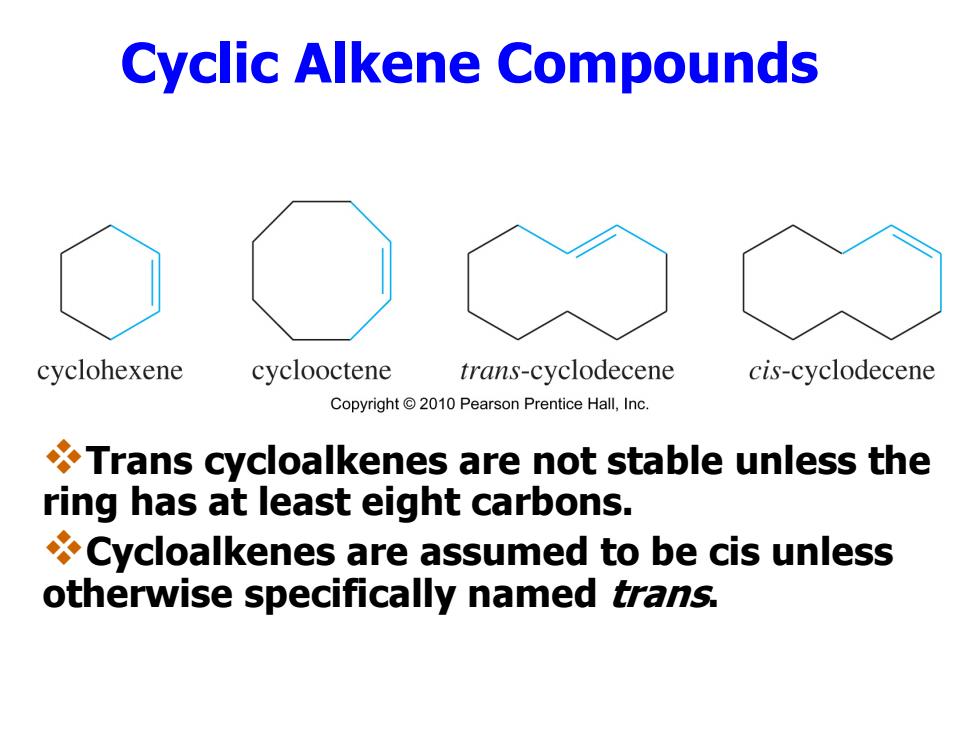
Cyclic Alkene Compounds cyclohexene cyclooctene trans-cyclodecene cis-cyclodecene Copyright2010 Pearson Prentice Hall,Inc. Trans cycloalkenes are not stable unless the ring has at least eight carbons. Cycloalkenes are assumed to be cis unless otherwise specifically named trans
Cyclic Alkene Compounds Trans cycloalkenes are not stable unless the ring has at least eight carbons. Cycloalkenes are assumed to be cis unless otherwise specifically named trans
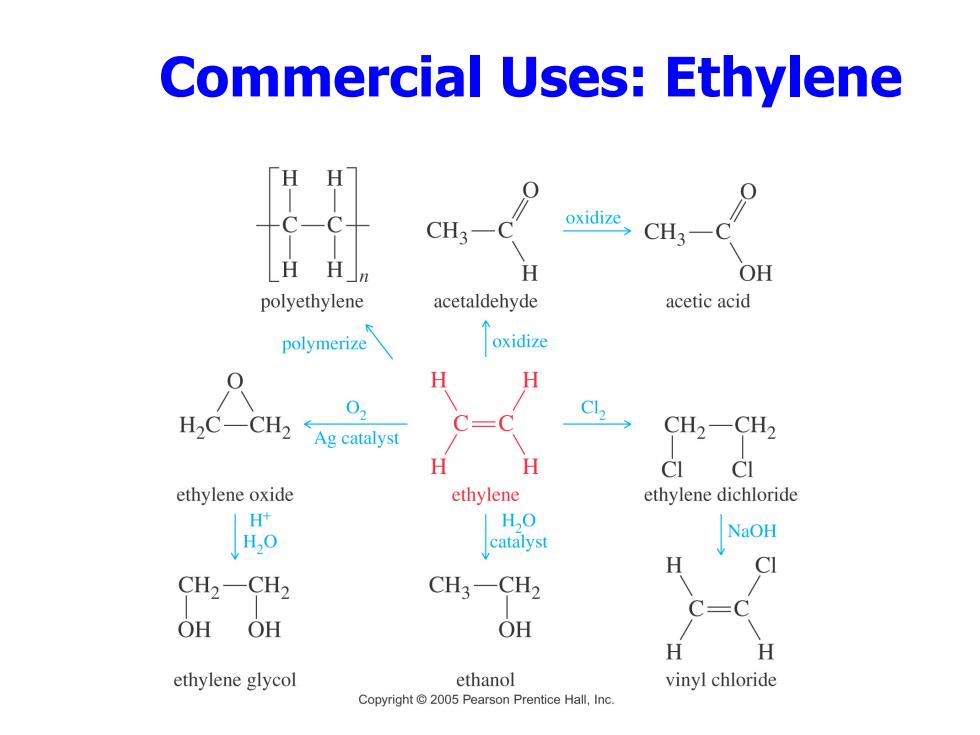
Commercial Uses:Ethylene CH3- oxidize CH3-C H H H OH polyethylene acetaldehyde acetic acid polymerize oxidize H H H2C-CH2 0 Ag catalyst CH2-CH2 H H CI CI ethylene oxide ethylene ethylene dichloride H+ HO H,0 catalyst NaOH H CH2-CH2 CH3-CH2 OH OH OH H ethylene glycol ethanol vinyl chloride Copyright 2005 Pearson Prentice Hall,Inc
Commercial Uses: Ethylene