0 花华院 Organic Chemistry,6h Edition L.G.Wade,Jr. Chapter 7 Alkyl Halides:Nucleophilic Substitution and Elimination By Junru Wang Email:wangjr07@163.com
By Junru Wang Email: wangjr07@163.com Chapter 7 Alkyl Halides: Nucleophilic Substitution and Elimination Organic Chemistry, 6 h Edition L. G. Wade, Jr

0环+ Key Notes Nucleophilic Substitution ●SN1 ●SN2 ■Elimination(E1,E2) ■Nucleophilicity .The rate of the SN2 reaction increases with the nucleophilic strength of the incoming nucleophile. OThe rate of the SN1 reaction is unaffected by the nature of the nucleophile ■Leaving group
Key Notes Nucleophilic Substitution SN1 SN2 Elimination(E1,E2) Nucleophilicity The rate of the SN2 reaction increases with the nucleophilic strength of the incoming nucleophile. The rate of the SN1 reaction is unaffected by the nature of the nucleophile Leaving group
0 Content 发牌院。 Preparation and physical properties Nucleophilic substitution Factors affecting SN2 versus SN1 reactions ■Elimination Elimination versus substitution Reactions of alkyl halides Organometallic reactions
Content Preparation and physical properties Nucleophilic substitution Factors affecting SN2 versus SN1 reactions Elimination Elimination versus substitution Reactions of alkyl halides Organometallic reactions
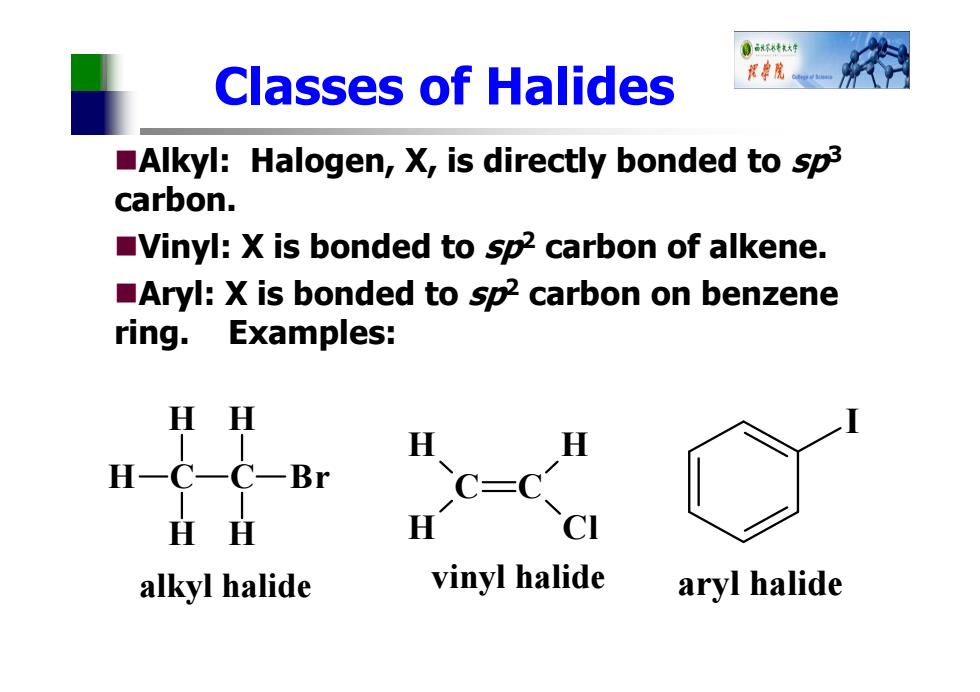
育不6特材车 Classes of Halides 花院 Alkyl:Halogen,X,is directly bonded to sp3 carbon. Vinyl:X is bonded to sp2 carbon of alkene. Aryl:X is bonded to sp2 carbon on benzene ring. Examples: HH H H一C一C一Br H H CI alkyl halide vinyl halide aryl halide
Classes of Halides Alkyl: Halogen, X, is directly bonded to sp3 carbon. Vinyl: X is bonded to sp2 carbon of alkene. Aryl: X is bonded to sp2 carbon on benzene ring. Examples: C H H H C H H Br alkyl halide C C H H H Cl vinyl halide I aryl halide

0礼不水牛大号 Classes of Alkyl Halides Methyl halides:only one C,CH2X Primary:C to which X is bonded has only one C-C bond. Secondary:C to which X is bonded has two C-C bonds. Tertiary:C to which X is bonded has three C-C bonds
Classes of Alkyl Halides Methyl halides: only one C, CH 3 X Primary: C to which X is bonded has only one C-C bond. Secondary: C to which X is bonded has two C-C bonds. Tertiary: C to which X is bonded has three C-C bonds