Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 17 18 Aromatic chemistry By Junru Wang Email:wangjr07@163.com 西北农林科技大学理学院
Chapter 17 & 18 Aromatic chemistry Organic Chemistry, 6th Edition L. G. Wade, Jr. By Junru Wang Email: wangjr07@163.com 西北农林科技大学理学院
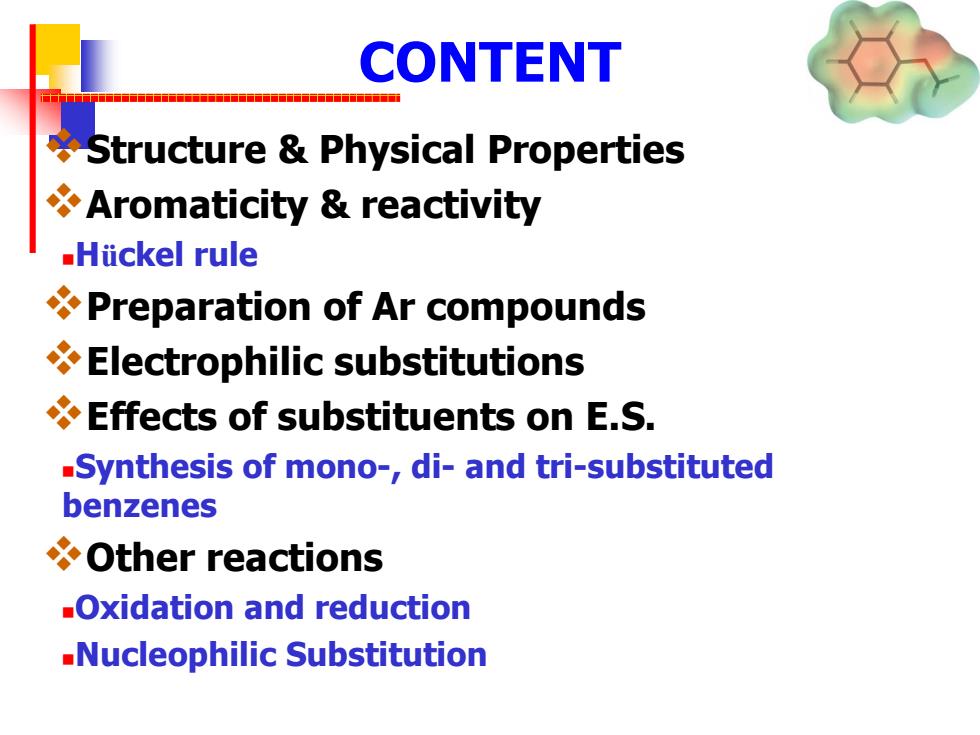
CONTENT Structure Physical Properties Aromaticity reactivity Hiickel rule Preparation of Ar compounds Electrophilic substitutions Effects of substituents on E.S. -Synthesis of mono-,di-and tri-substituted benzenes Other reactions -Oxidation and reduction -Nucleophilic Substitution
CONTENT Structure & Physical Properties Aromaticity & reactivity Hückel rule Preparation of Ar compounds Electrophilic substitutions Effects of substituents on E.S. Synthesis of mono-, di- and tri-substituted benzenes Other reactions Oxidation and reduction Nucleophilic Substitution
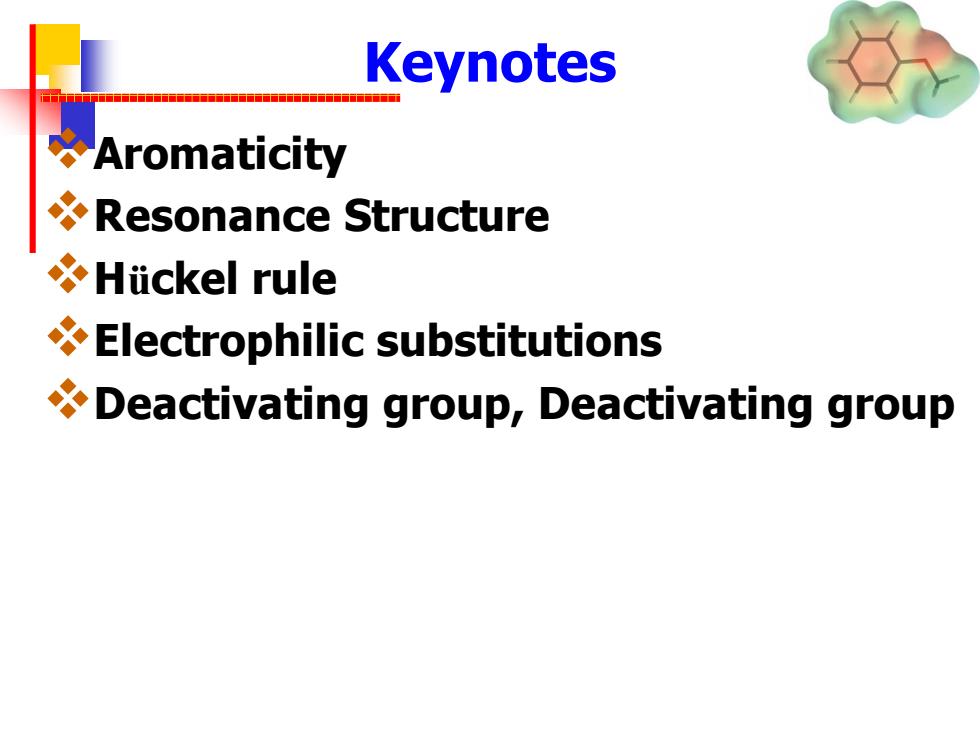
Keynotes Aromaticity Resonance Structure Hiickel rule Electrophilic substitutions Deactivating group,Deactivating group
Keynotes Aromaticity Resonance Structure Hückel rule Electrophilic substitutions Deactivating group, Deactivating group
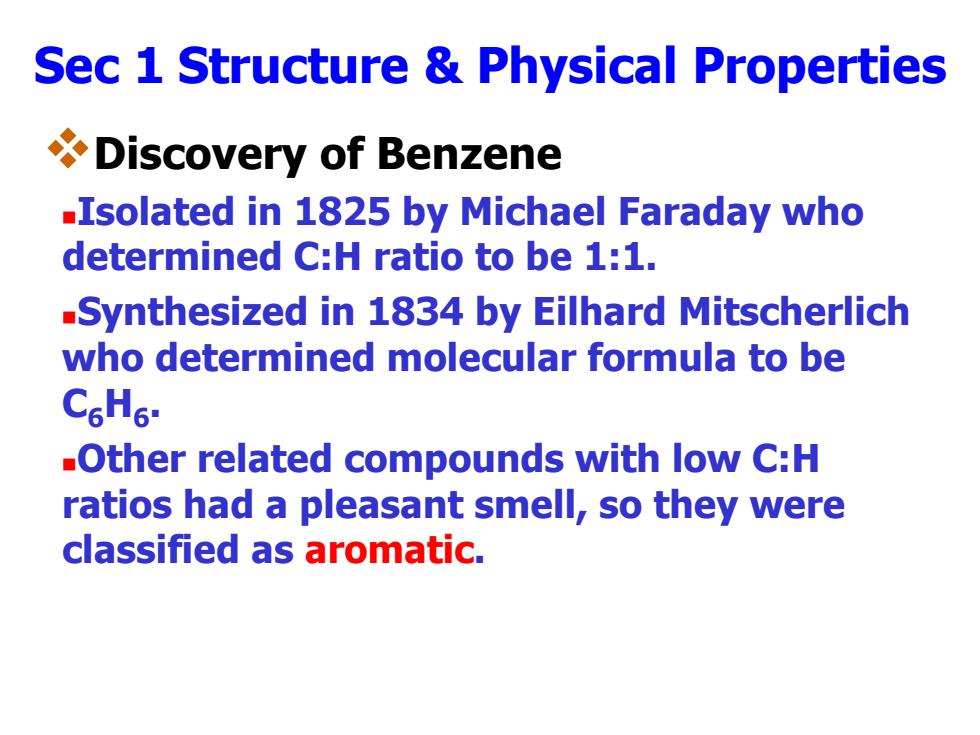
Sec 1 Structure Physical Properties Discovery of Benzene -Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. -Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6H6. -Other related compounds with low C:H ratios had a pleasant smell,so they were classified as aromatic
Sec 1 Structure & Physical Properties Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6 H6 . Other related compounds with low C:H ratios had a pleasant smell, so they were classified as aromatic
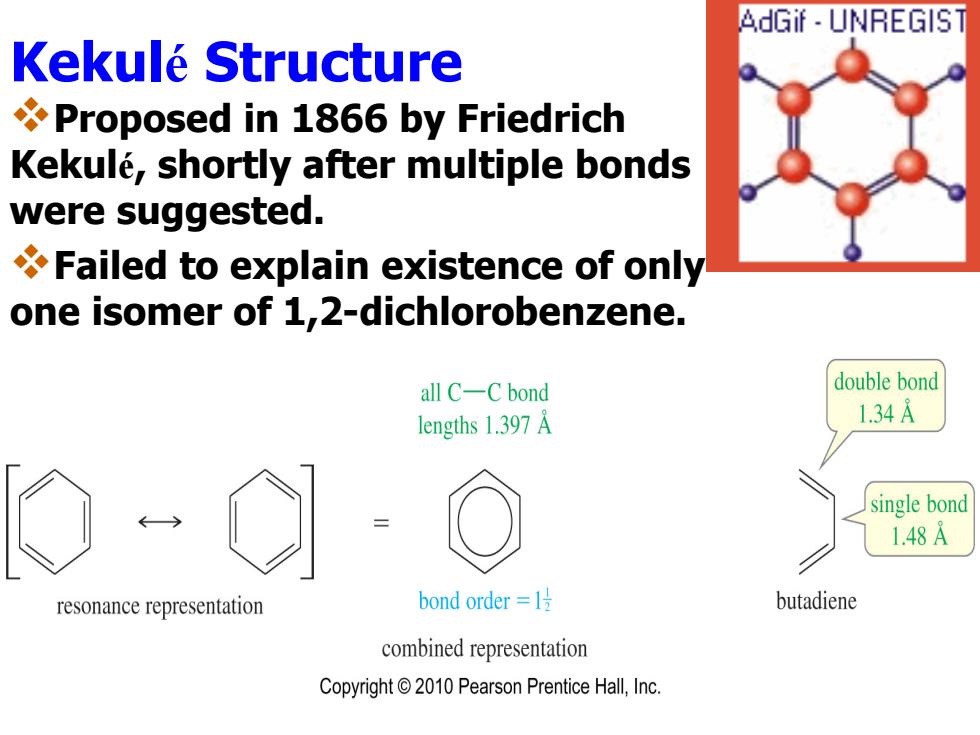
AdGif UNREGIST Kekule Structure Proposed in 1866 by Friedrich Kekule,shortly after multiple bonds were suggested. Failed to explain existence of only one isomer of 1,2-dichlorobenzene. all C-C bond double bond lengths 1.397 A 1.34A single bond 1.48A resonance representation bond order=l号 butadiene combined representation Copyright 2010 Pearson Prentice Hall,Inc
Kekulé Structure Proposed in 1866 by Friedrich Kekul é, shortly after multiple bonds were suggested. Failed to explain existence of only one isomer of 1,2-dichlorobenzene