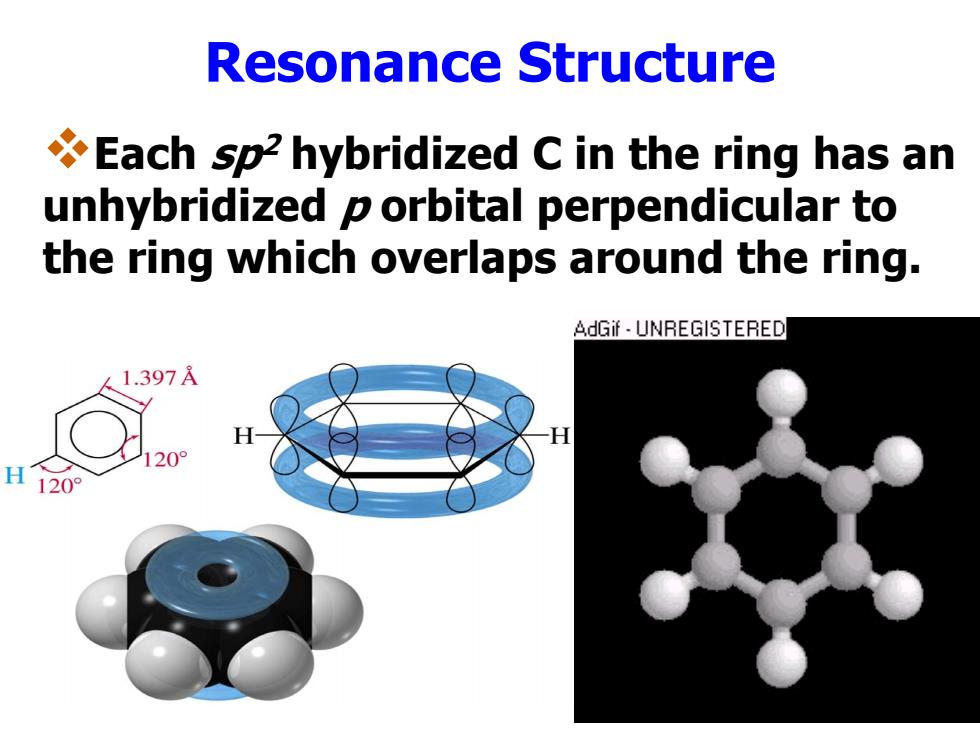
Resonance Structure Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. AdGif UNREGISTERED 1.397A 20 H 120°
Resonance Structure Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring
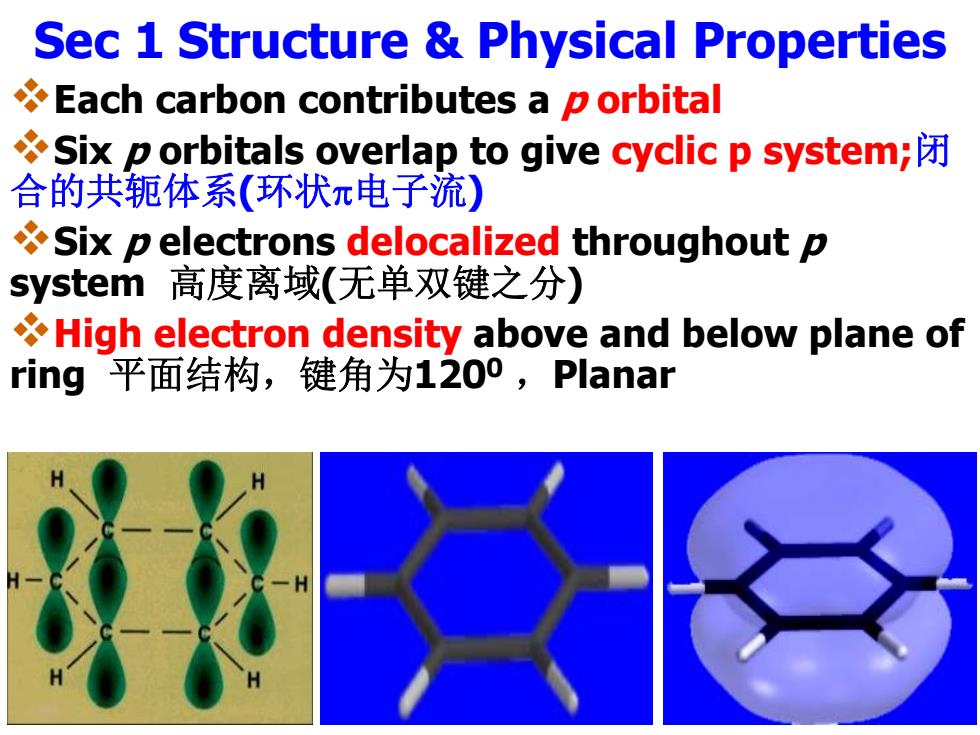
Sec 1 Structure Physical Properties Each carbon contributes a p orbital Six p orbitals overlap to give cyclic p system; 合的共轭体系(环状π电子流) Six p electrons delocalized throughout p system高度离域(无单双键之分) High electron density above and below plane of ring平面结构,键角为1200,Planar
Sec 1 Structure & Physical Properties Each carbon contributes a p orbital Six p orbitals overlap to give cyclic p system; 闭 合的共轭体系 (环状 电子流 ) Six p electrons delocalized throughout p system 高度离域 (无单双键之分 ) High electron density above and below plane of ring 平面结构,键角为1200 ,Planar
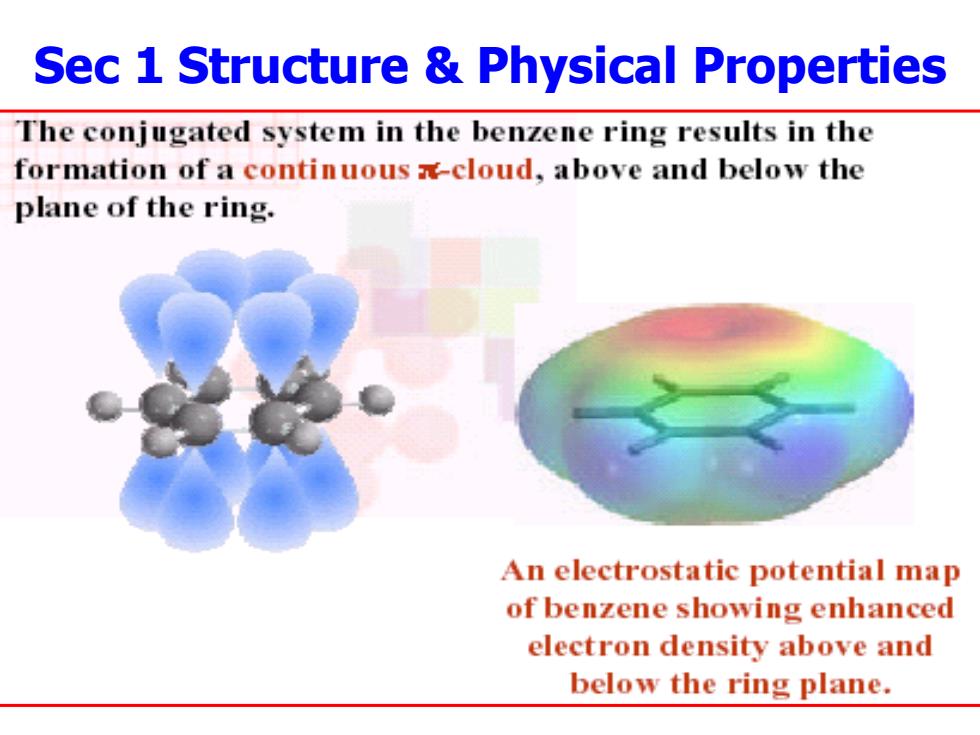
Sec 1 Structure Physical Properties The conjugated system in the benzene ring results in the formation of a continuous -cloud,above and below the plane of the ring. An electrostatic potential map of benzene showing enhanced electron density above and below the ring plane
Sec 1 Structure & Physical Properties
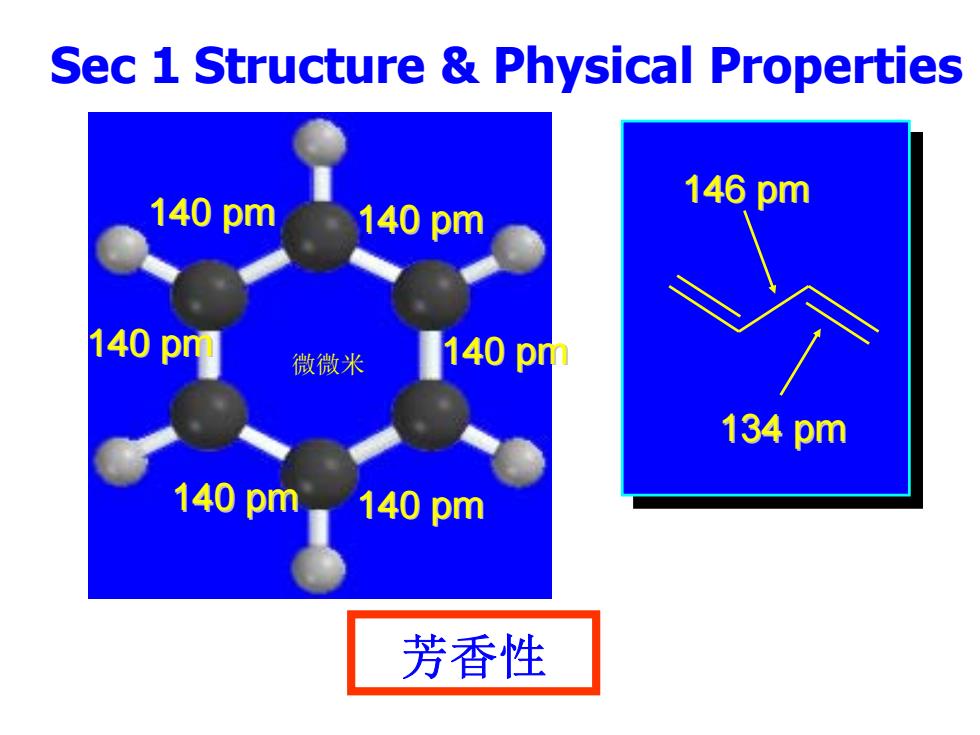
Sec 1 Structure Physical Properties 140pm 146pm 140pm 140pm 微微米 140pm 134pm 140pm 140pm 芳香性
Sec 1 Structure & Physical Properties 芳香性 140 pm 140 pm 140 pm 140 pm 140 pm 140 pm 146 pm 134 pm 微微米
Unusual Reactions Alkene KMnO4>diol (addition) Benzene KMnO4->no reaction. Alkene Br2/CCl4>dibromide (addition) -Benzene Br2/CCl4->no reaction. With FeCl3 catalyst,Br2 reacts with benzene to form bromobenzene HBr (substitution!). -Double bonds remain
Unusual Reactions Alkene + KMnO4 diol (addition) Benzene + KMnO4 no reaction. Alkene + Br2 /CCl4 dibromide (addition) Benzene + Br2 /CCl4 no reaction. With FeCl3 catalyst, Br2 reacts with benzene to form bromobenzene + HBr (substitution!). Double bonds remain