有机化学oRGAnIc©EmISTRY 盖讲:王德儒87092829(0) 理学院寇化素理科接2层206 Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 10 AlRynes Key Notes Electronic Structure;Acidity;Terminal alkynes Homework.10-22:10-23:(P274)
Chapter 10 Alkynes Organic Chemistry, 6th Edition L. G. Wade, Jr. Key Notes Electronic Structure; Acidity; Terminal alkynes 有机化学ORGANIC CHEMISTRY 主讲:王俊儒 87092829(O) 理学院应化系理科楼2层C206 Homework:10-22; 10-23; (P274)
音秋标特大对 CONTENTS Properties Preparation ■Reactions of alkynes Electrophilic additions to alkynes Reduction Oxidation of alkynes OSpecial reactions of terminal alkynes
CONTENTS ◼Properties ◼Preparation ◼Reactions of alkynes ⚫Electrophilic additions to alkynes ⚫Reduction & Oxidation of alkynes ⚫Special reactions of terminal alkynes
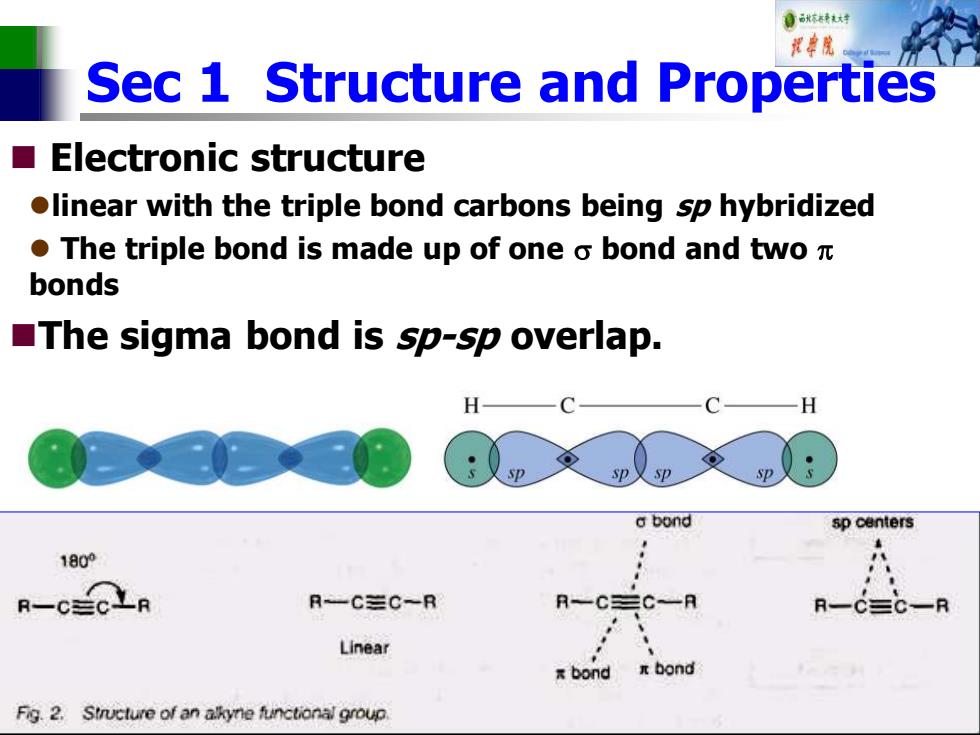
自秋转达对 报中院 Sec 1 Structure and Properties Electronic structure olinear with the triple bond carbons being sp hybridized ●The triple bond is made up of one o bond and twoπ bonds The sigma bond is sp-sp overlap. H a bond sp centers 1800 R-CECLR R-C三C~R Linear Fig.2.Structure of an akyne functional group
Sec 1 Structure and Properties ◼ Electronic structure ⚫linear with the triple bond carbons being sp hybridized ⚫ The triple bond is made up of one bond and two bonds ◼The sigma bond is sp-sp overlap
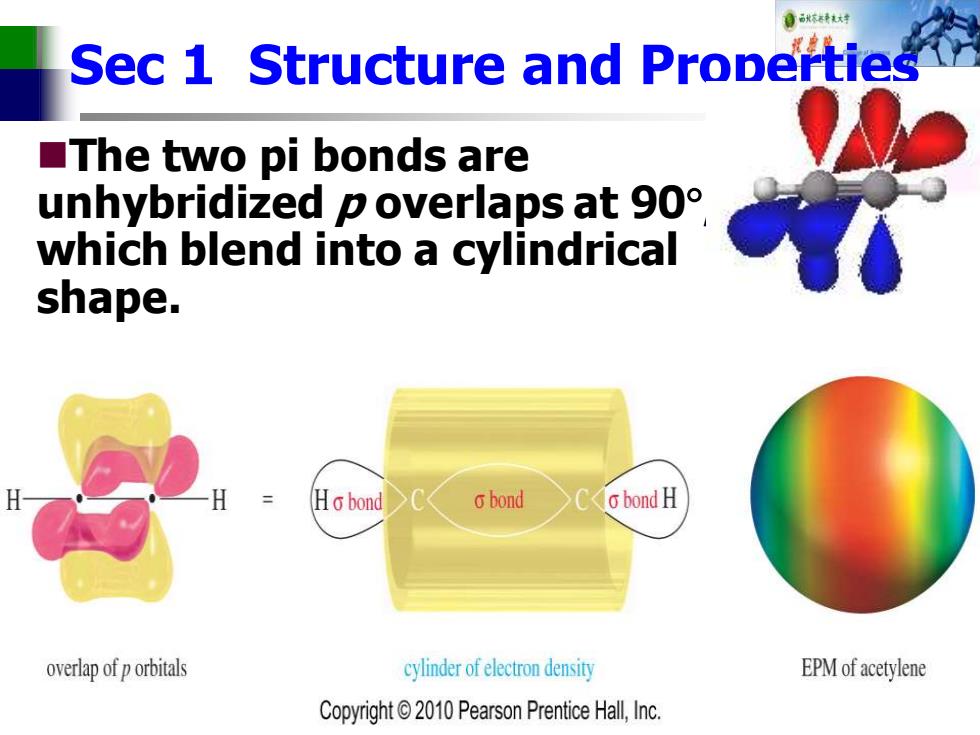
Sec 1 S Structure and Properties ■The two pi bonds are unhybridized p overlaps at 90 which blend into a cylindrical shape. Ho bond 6bond o bond H overlap of p orbitals cylinder of electron density EPM of acetylene Copyright2010 Pearson Prentice Hall,Inc
Sec 1 Structure and Properties ◼The two pi bonds are unhybridized p overlaps at 90 , which blend into a cylindrical shape
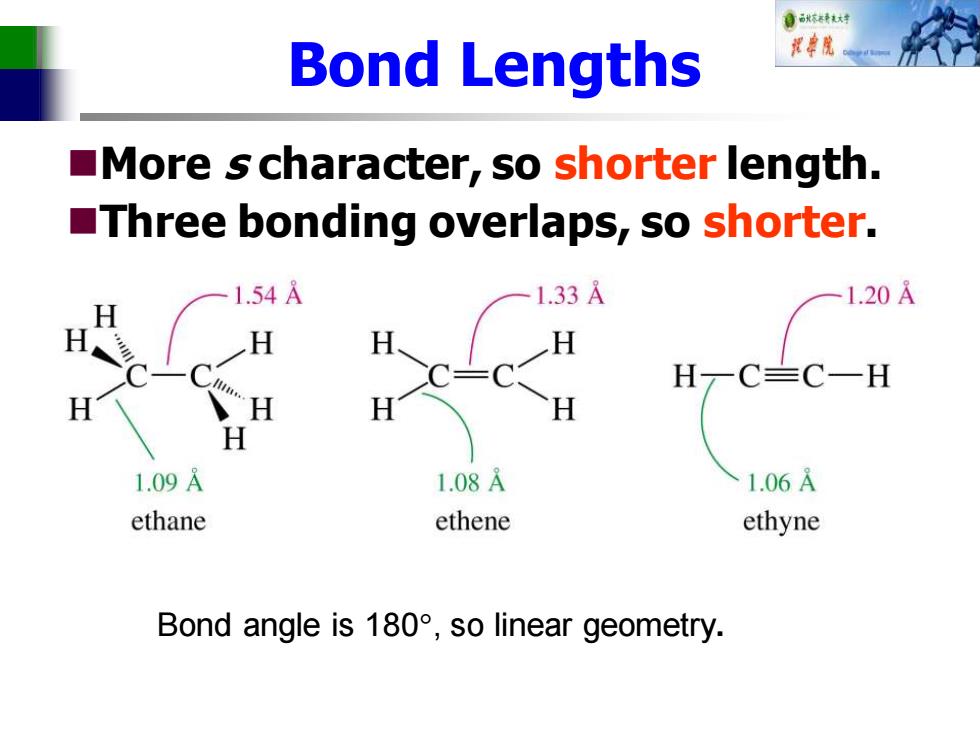
自秋不转大对 Bond Lengths More s character,so shorter length. Three bonding overlaps,so shorter. 1.54A 1.33 1.20A H H H H一CC一H H H H H 1.09A 1.08A 1.06A ethane ethene ethyne Bond angle is180°,so linear geometry
Bond Lengths ◼More s character, so shorter length. ◼Three bonding overlaps, so shorter. Bond angle is 180, so linear geometry