
Organic Chemistry with BiologicalApplications,3Edition John McMurry Chapter 03 Organic compounds: alkanes and their stereochemistry Alkanes are relatively unreactive with onty a minor role in a few biological processes. This chap will provide some of 3D aspects of molecules,with a topic of particular importance in understanding biological organic chemistry
Chapter 03 Organic compounds : alkanes and their stereochemistry Organic Chemistry with Biological Applications, 3 rd Edition John McMurry Alkanes are relatively unreactive with only a minor role in a few biological processes. This chap will provide some of 3D aspects of molecules, with a topic of particular importance in understanding biological organic chemistry

Organic Chemistry with BiologicalApplications,3rEdition John McMurry Chapter 03 Organic compounds: alkanes and their stereochemistry Key Notes Conformation;Conformers;Isomer Radical RXN
Chapter 03 Organic compounds : alkanes and their stereochemistry Key Notes Conformation; Conformers; Isomer; Radical RXN Organic Chemistry with Biological Applications, 3 rd Edition John McMurry
Main Contents Alkanes and Alkane Isomers ■Alkyl Groups Properties of Alkanes ■Conformations √Ethane √Other alkanes A membrane channel protein that conducts K+ions across cell membranes
Main Contents ◼Alkanes and Alkane Isomers ◼Alkyl Groups ◼Properties of Alkanes ◼Conformations ✓Ethane ✓Other alkanes A membrane channel protein that conducts K+ ions across cell membranes

Sec 1 Alkanes and Alkane Isomers Alkanes often described as saturated hydrocarbons Hydrocarbons Contain only carbon and hydrogen Saturated Contain maximum possible number of hydrogens per carbon and have only C-C and C-H single bonds Alkanes occasionally referred to as aliphatic compounds,a name derived from the Greek word aleiphas,meaning "fat" Fatty acyl Glycerol Stearoyl (stearic acid) CH2OCCH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH3 Oleoyl (oleic acid) CHOCCH2CH2CH2CH2CH2CH2CH2CH-CHCH2CH2CH2CH2CH2CH2CH2CH3 Linoleoyl (linoleic acid) CH2OCCH2CH2CH2CH2CH2CH2CH2CH=CHCH2CH-CHCH2CH2CH2CH2CH3 A triacylglycerol Cengnge Leaming All Righes Reporved
Alkanes often described as saturated hydrocarbons ▪ Hydrocarbons Contain only carbon and hydrogen ▪ Saturated ▪ Contain maximum possible number of hydrogens per carbon and have only C-C and C-H single bonds Alkanes occasionally referred to as aliphatic compounds, a name derived from the Greek word aleiphas, meaning “fat” Sec 1 Alkanes and Alkane Isomers
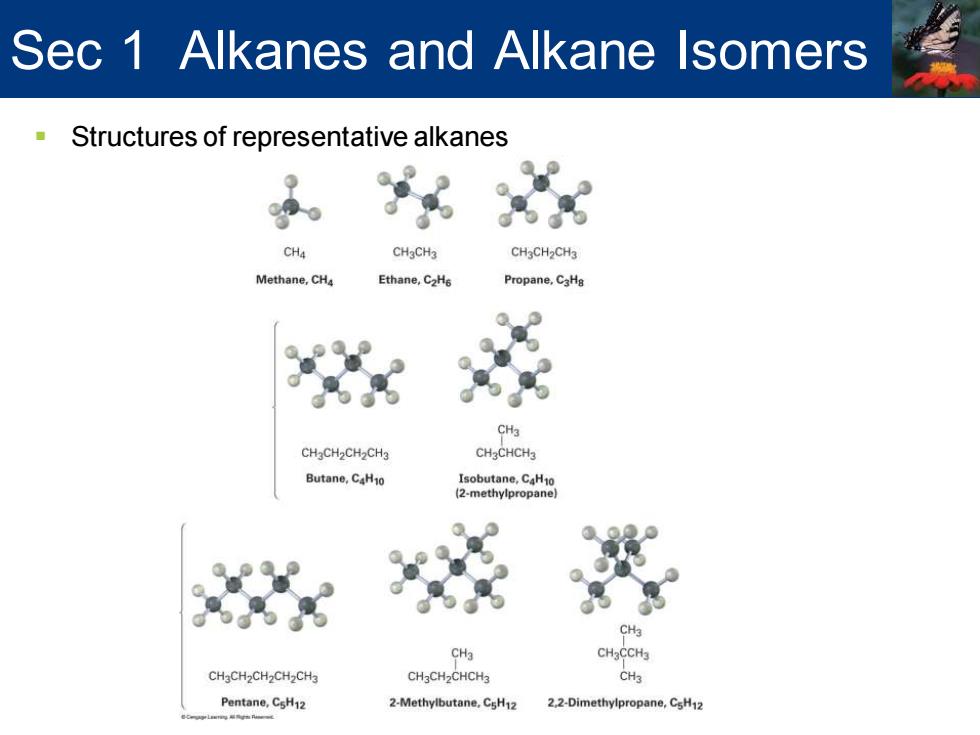
Sec 1 Alkanes and Alkane Isomers Structures of representative alkanes CH4 CHaCH3 CH3CH2CH3 Methane,CHa Ethane,C2H6 Propane,CaHg CH3 CHgCH2CH2CH3 CH2CHCH3 Butane,C4H10 Isobutane,CaH10 2-methylpropane】 C CH3 CHaCCHa CH3CH2CH2CH2CH3 CH3CH2CHCH3 CH3 Pentane,CsH12 2-Methylbutane.CsH12 2.2-Dimethylpropane,CsH12
▪ Structures of representative alkanes Sec 1 Alkanes and Alkane Isomers