
Solved Problem 4-1 Give a systematic (IUPAC)name for the following compound. The longest carbon chain contains eight carbon atoms,so this compound is named as an octane.Numbering from left to right gives the first branch on C2;numbering from right to left gives the first branch on C3,so we number from left to riaht. CH 8 CH一CH CH,CH, C CH,- ECH-4CH-CH,-CH-CH3 3 CH. CH CH Copyright2010 Pearson Prentice Hall,Inc 4-isopropyl-2,2,3,6-tetramethyloctane
The longest carbon chain contains eight carbon atoms, so this compound is named as an octane. Numbering from left to right gives the first branch on C2; numbering from right to left gives the first branch on C3, so we number from left to right. Solved Problem 4-1 4-isopropyl-2,2,3,6-tetramethyloctane Give a systematic (IUPAC) name for the following compound

SEC 1 Physical Properties Solubility:hydrophobic Density:less than 1 g/mL Boiling points increase with increasing carbons (little less for branched chains). Melting points increase with increasing carbons (less for odd-number of carbons)
SEC 1 Physical Properties ◼Solubility: hydrophobic ◼Density: less than 1 g/mL ◼Boiling points increase with increasing carbons (little less for branched chains). ◼Melting points increase with increasing carbons (less for odd-number of carbons)

自秋不转大对 Boiling Points of Alkanes ■ Branched alkanes have less surface area contact,so weaker intermolecular forces. 400 300 CH3-(CH2)n-CH3 ()uod 3u!oq 200 n-alkanes 100 0 CH-(CH2)CH3 100 isoalkanes CH3 -200 5 10 15 20 number of carbon atoms
Boiling Points of Alkanes ◼Branched alkanes have less surface area contact, so weaker intermolecular forces
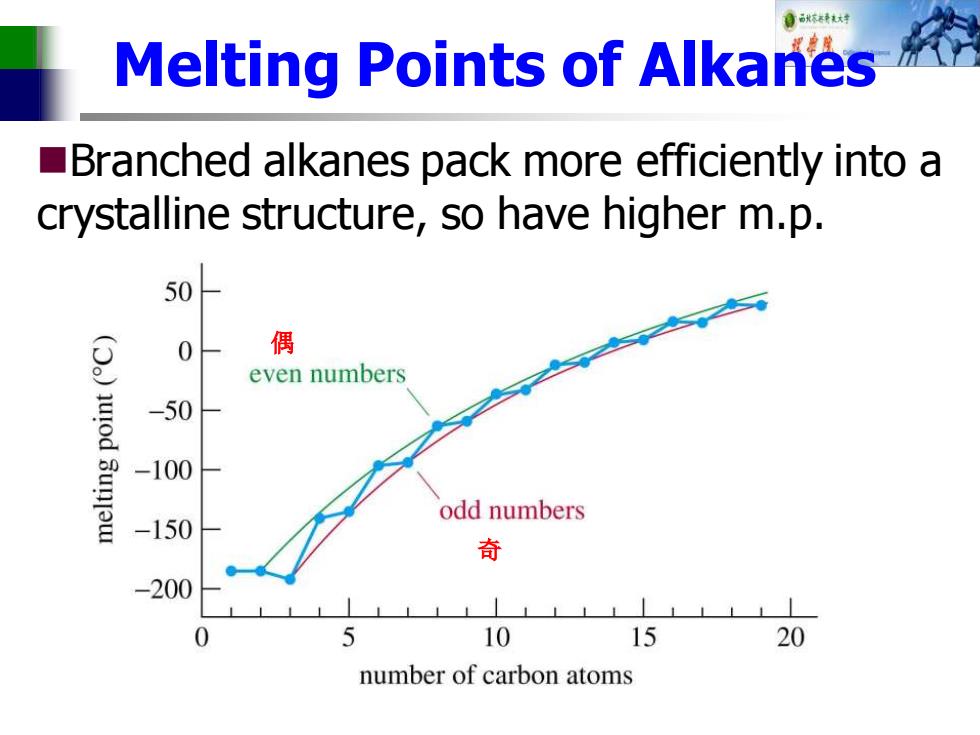
自秋不特大对 Melting Points of Alkanes Branched alkanes pack more efficiently into a crystalline structure,so have higher m.p. 50 偶 even numbers -50 -100 odd numbers -150 奇 -200 0 5 10 15 20 number of carbon atoms
Melting Points of Alkanes ◼Branched alkanes pack more efficiently into a crystalline structure, so have higher m.p. 偶 奇
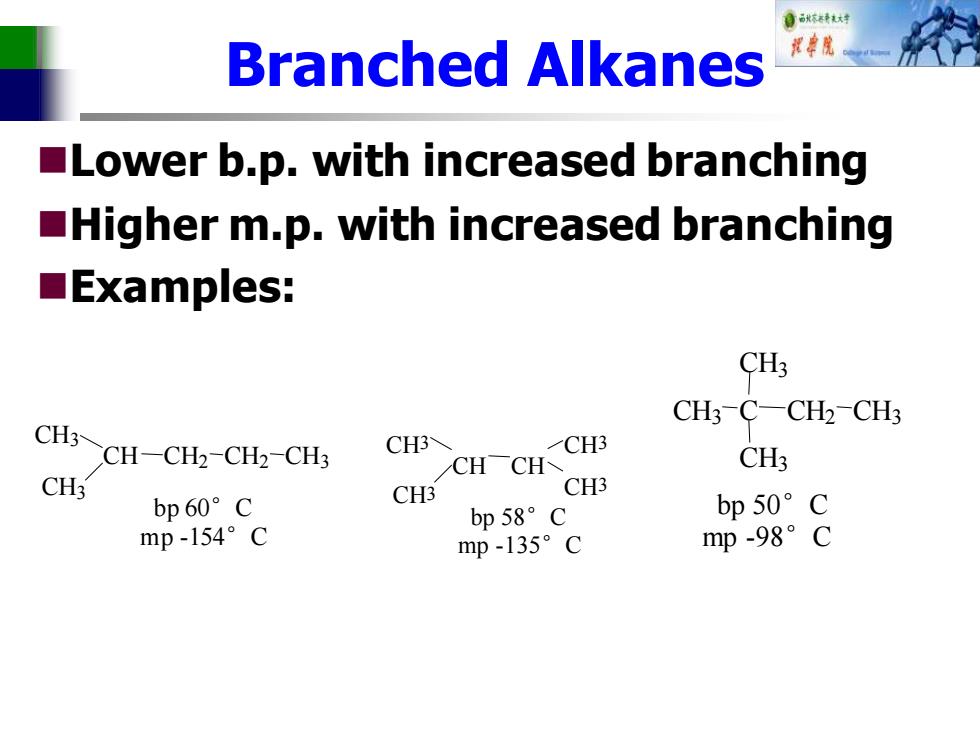
自秋东精秋对 Branched Alkanes Lower b.p.with increased branching Higher m.p.with increased branching ■Examples: CH3 CH3-C-CH2-CH3 CH3~CH-CH2-CH2-CHs CH3 CH3 CHCH CH3 CH3 bp60°C CH3 CH3 bp58°C bp50°C mp-154°C mp-135°C mp-98°C
Branched Alkanes ◼Lower b.p. with increased branching ◼Higher m.p. with increased branching ◼Examples: H CH3 CH CH3 CH2 CH2 CH3 bp 60°C mp -154°C CH3 CH CH3 CH CH3 CH3 bp 58°C mp -135°C bp 50°C mp -98°C CH3 C C 3 CH3 CH2 CH3