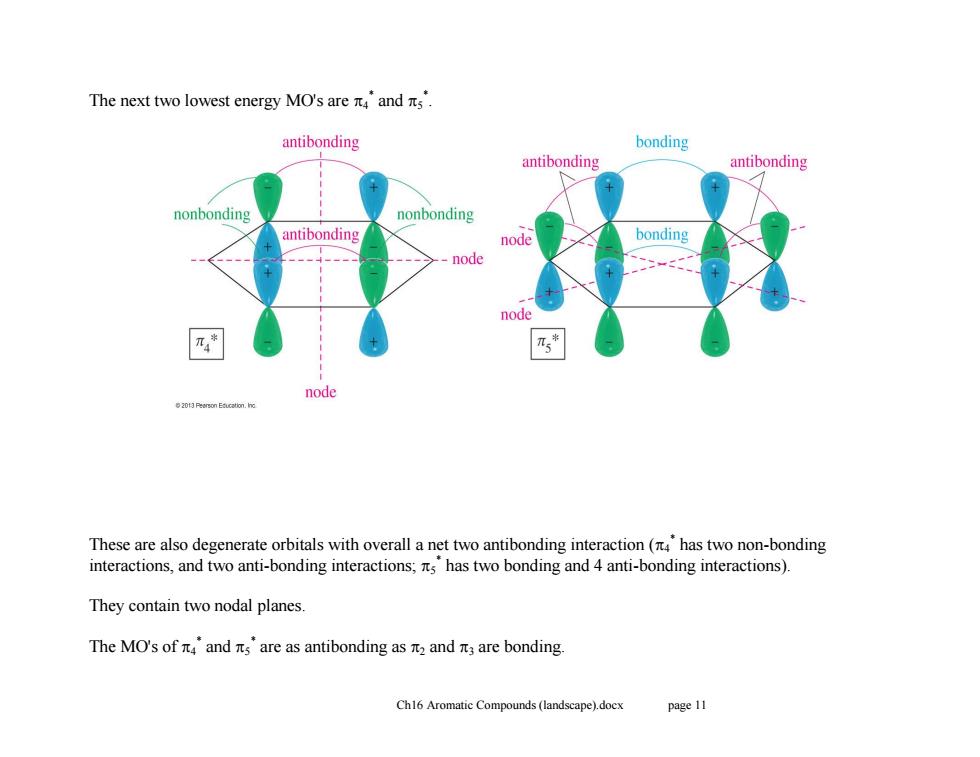
The next two lowest energy MO'sare元4and元s. antibonding bonding antibonding antibonding nonbonding nonbonding antibonding node bonding -node node 乃* node 2013 Peerson Education.hno These are also degenerate orbitals with overall a net two antibonding interaction(has two non-bonding interactions,and two anti-bonding interactions;ts has two bonding and 4 anti-bonding interactions). They contain two nodal planes. The MO'sof元4and元sare as antibonding as2and元3 are bonding. Ch16 Aromatic Compounds(landscape).docx page 11
Ch16 Aromatic Compounds (landscape).docx page 11 The next two lowest energy MO's are 4 * and 5 * . These are also degenerate orbitals with overall a net two antibonding interaction (4 * has two non-bonding interactions, and two anti-bonding interactions; 5 * has two bonding and 4 anti-bonding interactions). They contain two nodal planes. The MO's of 4 * and 5 * are as antibonding as 2 and 3 are bonding
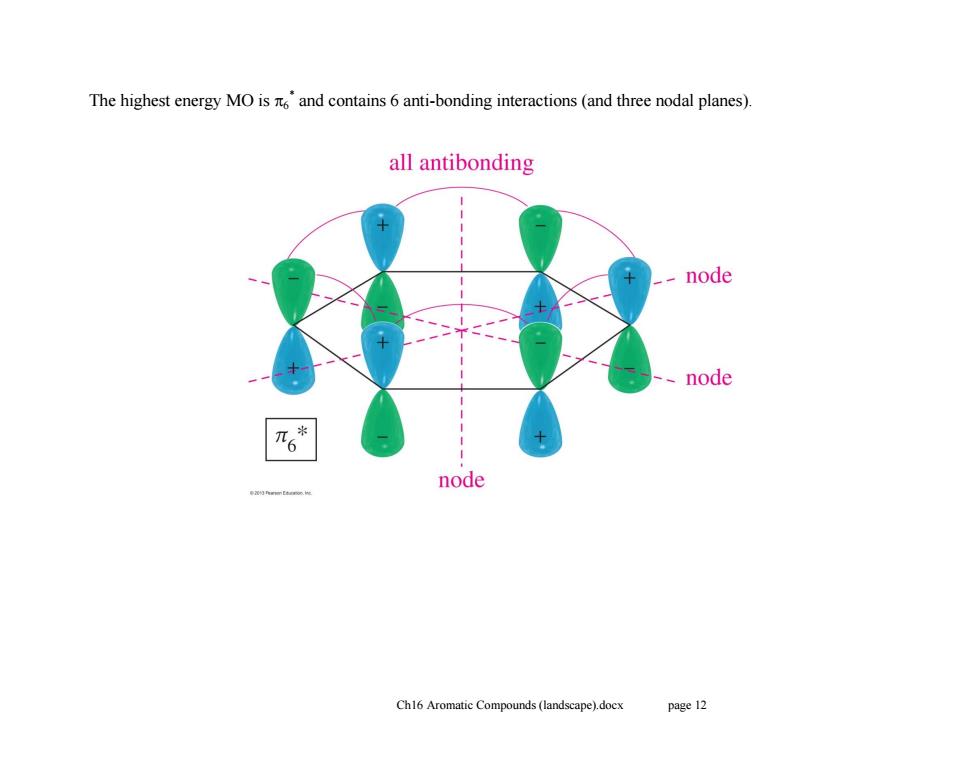
The highest energy MO is and contains 6 anti-bonding interactions(and three nodal planes). all antibonding -node 、node n6* node Ch16 Aromatic Compounds(landscape).docx page 12
Ch16 Aromatic Compounds (landscape).docx page 12 The highest energy MO is 6 * and contains 6 anti-bonding interactions (and three nodal planes)
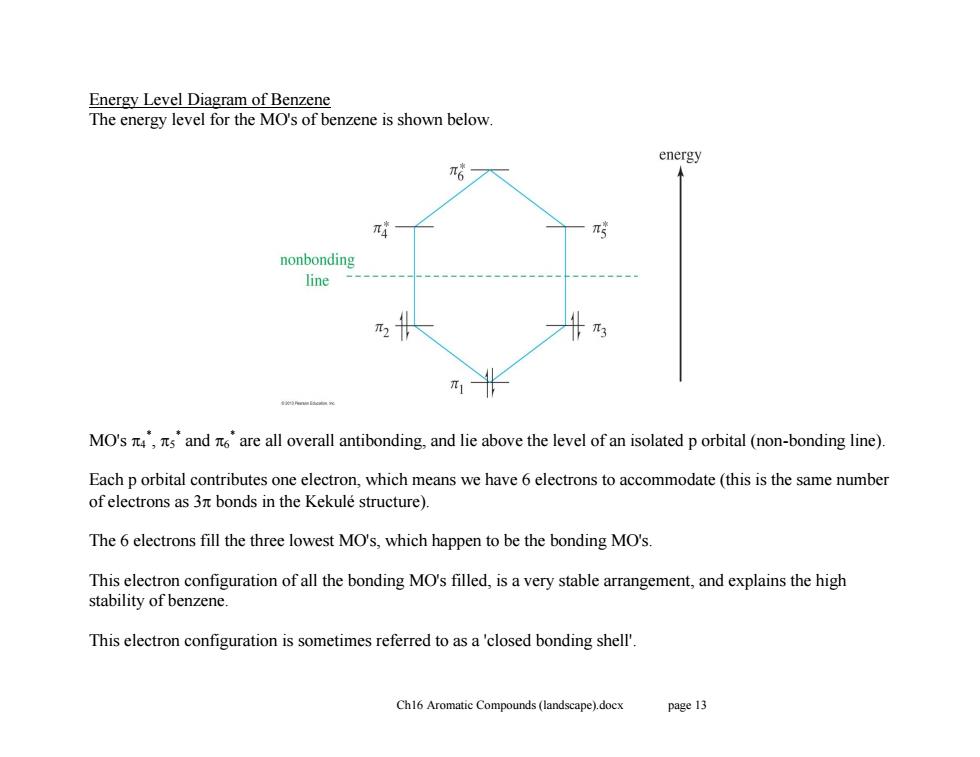
Energy Level Diagram of Benzene The energy level for the MO's of benzene is shown below energy π6 nonbonding line π3 π1 MO's,sand are all overall antibonding,and lie above the level of an isolated p orbital (non-bonding line). Each p orbital contributes one electron,which means we have 6 electrons to accommodate(this is the same number of electrons as 3nt bonds in the Kekule structure). The 6 electrons fill the three lowest MO's,which happen to be the bonding MO's. This electron configuration of all the bonding MO's filled,is a very stable arrangement,and explains the high stability of benzene This electron configuration is sometimes referred to as a'closed bonding shell'. Ch16 Aromatic Compounds(landscape).docx page 13
Ch16 Aromatic Compounds (landscape).docx page 13 Energy Level Diagram of Benzene The energy level for the MO's of benzene is shown below. MO's 4 * , 5 * and 6 * are all overall antibonding, and lie above the level of an isolated p orbital (non-bonding line). Each p orbital contributes one electron, which means we have 6 electrons to accommodate (this is the same number of electrons as 3 bonds in the Kekulé structure). The 6 electrons fill the three lowest MO's, which happen to be the bonding MO's. This electron configuration of all the bonding MO's filled, is a very stable arrangement, and explains the high stability of benzene. This electron configuration is sometimes referred to as a 'closed bonding shell
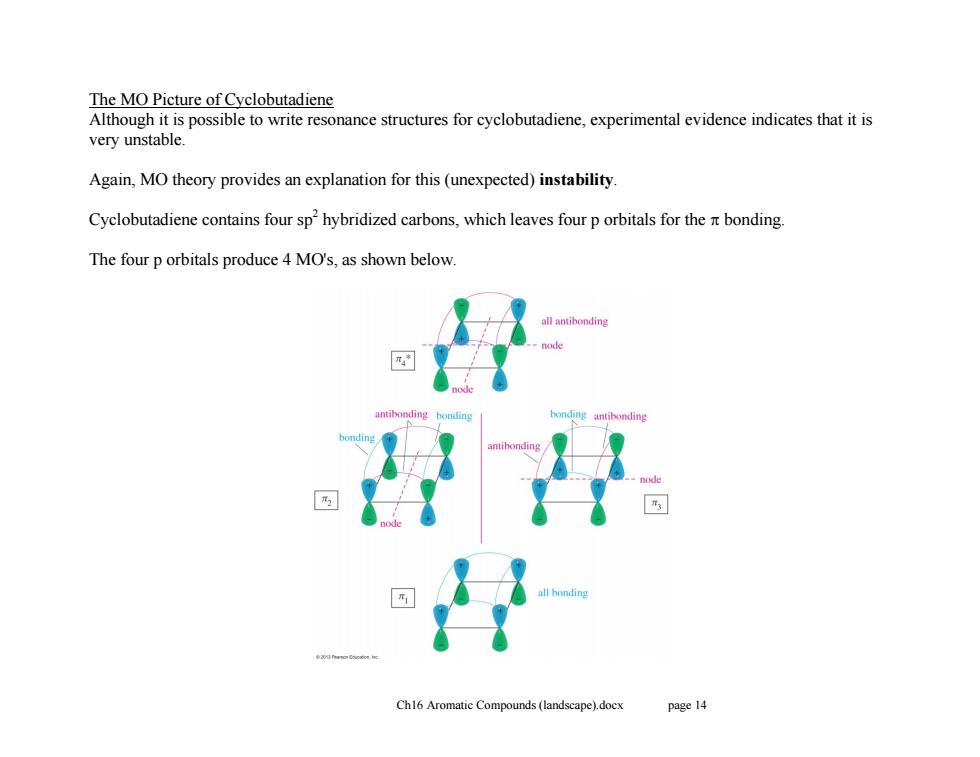
The MO Picture of Cyclobutadiene Although it is possible to write resonance structures for cyclobutadiene,experimental evidence indicates that it is very unstable. Again,MO theory provides an explanation for this (unexpected)instability Cyclobutadiene contains four sphybridized carbons,which leaves four p orbitals for the bonding. The four p orbitals produce 4 MO's,as shown below. Ch16 Aromatic Compounds (landscape).docx page 14
Ch16 Aromatic Compounds (landscape).docx page 14 The MO Picture of Cyclobutadiene Although it is possible to write resonance structures for cyclobutadiene, experimental evidence indicates that it is very unstable. Again, MO theory provides an explanation for this (unexpected) instability. Cyclobutadiene contains four sp2 hybridized carbons, which leaves four p orbitals for the bonding. The four p orbitals produce 4 MO's, as shown below