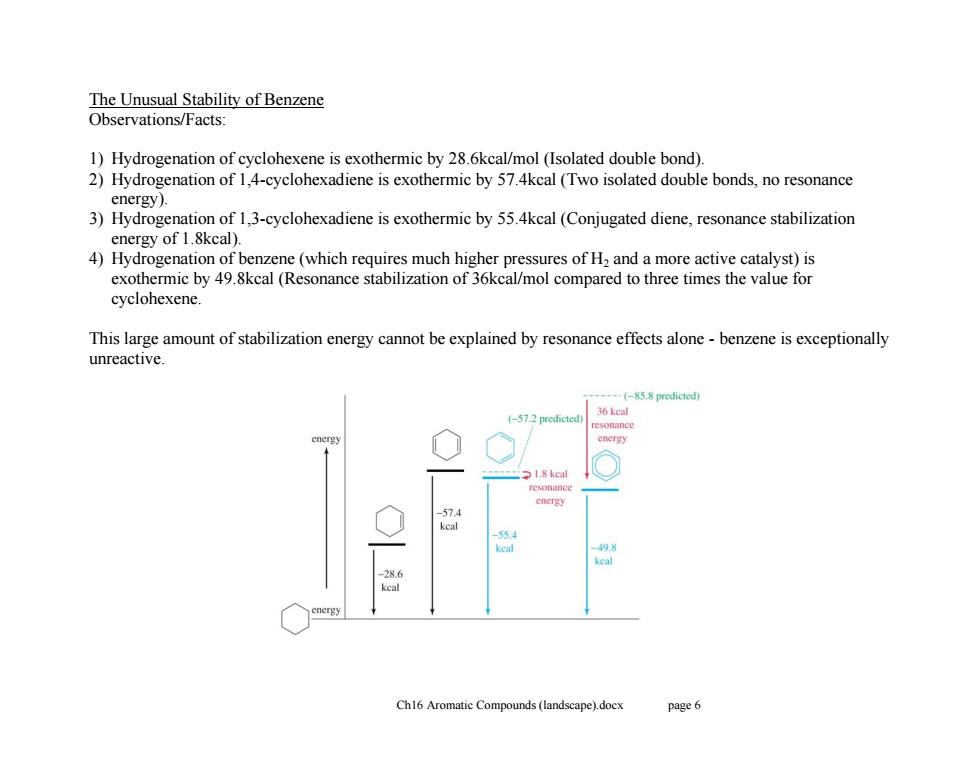
The Unusual Stability of Benzene Observations/Facts: 1)Hydrogenation of cyclohexene is exothermic by 28.6kcal/mol (Isolated double bond). 2)Hydrogenation of 1,4-cyclohexadiene is exothermic by 57.4kcal(Two isolated double bonds,no resonance energy). 3)Hydrogenation of 1,3-cyclohexadiene is exothermic by 55.4kcal(Conjugated diene,resonance stabilization energy of 1.8kcal). 4)Hydrogenation of benzene(which requires much higher pressures of H2 and a more active catalyst)is exothermic by 49.8kcal (Resonance stabilization of 36kcal/mol compared to three times the value for cyclohexene This large amount of stabilization energy cannot be explained by resonance effects alone-benzene is exceptionally unreactive. (-85.8 predictedy 36 kcal (-57.2 predicted) 1.8 kcal 55 keal 28.6 kcal Ch16 Aromatic Compounds (landscape).docx page 6
Ch16 Aromatic Compounds (landscape).docx page 6 The Unusual Stability of Benzene Observations/Facts: 1) Hydrogenation of cyclohexene is exothermic by 28.6kcal/mol (Isolated double bond). 2) Hydrogenation of 1,4-cyclohexadiene is exothermic by 57.4kcal (Two isolated double bonds, no resonance energy). 3) Hydrogenation of 1,3-cyclohexadiene is exothermic by 55.4kcal (Conjugated diene, resonance stabilization energy of 1.8kcal). 4) Hydrogenation of benzene (which requires much higher pressures of H2 and a more active catalyst) is exothermic by 49.8kcal (Resonance stabilization of 36kcal/mol compared to three times the value for cyclohexene. This large amount of stabilization energy cannot be explained by resonance effects alone - benzene is exceptionally unreactive

Failures of the Resonance Picture for Aromatics If having these identical resonance structures were the sole cause of this pronounced stability,then ALL structures with conjugated systems of alternating double and single bonds should show analogous enhanced stabilities. These cyclic hydrocarbons with alternating double and single carboncarbon bonds are called Annulenes. 回 [4]Annulene [6]Annulene [8]Annulene [10]Annulene Benzene is the 6 membered annulene,and is called [6]annulene. For the double bonds to be totally conjugated,the molecule must be planar so that the p orbitals of the bonds can overlap. However,molecules like cyclobutadiene and cyclo-octatetraene DO NOT exhibit this increased stability-in fact quite the opposite! Cyclobutadiene has never been isolated and purified because it is so unstable-it reacts with itself to form dimers even at low temperatures. Cyclo-octatetraene has been shown to not exist in a planar structure,but instead it adopts a'tub'like conformation. Ch16 Aromatic Compounds(landscape).docx page 7
Ch16 Aromatic Compounds (landscape).docx page 7 Failures of the Resonance Picture for Aromatics If having these identical resonance structures were the sole cause of this pronounced stability, then ALL structures with conjugated systems of alternating double and single bonds should show analogous enhanced stabilities. These cyclic hydrocarbons with alternating double and single carbon carbon bonds are called Annulenes. Benzene is the 6 membered annulene, and is called [6] annulene. For the double bonds to be totally conjugated, the molecule must be planar so that the p orbitals of the bonds can overlap. However, molecules like cyclobutadiene and cyclo-octatetraene DO NOT exhibit this increased stability - in fact quite the opposite! Cyclobutadiene has never been isolated and purified because it is so unstable - it reacts with itself to form dimers even at low temperatures. Cyclo-octatetraene has been shown to not exist in a planar structure, but instead it adopts a 'tub' like conformation

MO's of Benzene Benzene's extra stability cannot be explained by resonance alone,and so we must turn to Molecular Orbital theory for a fuller answer. Benzene has 6 planar sp'carbons,and therefore each carbon has an unhybridized p orbital. These p orbitals are perfectly aligned for overlap(ie.bonding,just like for a bond) These p orbitals create a continuous ring of orbitals above and below the plane of the carbon atoms. The 6 overlapping p orbitals create a cyclic system of molecular orbitals(i.e.a three dimensional system). Even though we have only seen two dimensional MO's previously(ethene,allyl systems,the same basic rules apply. 1)Six p orbitals are used in the benzene system,therefore six MO's are created. 2)The lowest energy MO is entirely bonding(constructive overlap between all adjacent p orbitals,no nodes). 3)The number of nodes increases as the MO's increase in energy. 4)The MO's must be divided between bonding and antibonding,with the possibility on non-bonding MO's in some cases. The 6 MO's for benzene can be drawn either in 2D or 3D projections. Ch16 Aromatic Compounds (landscape).docx page 8
Ch16 Aromatic Compounds (landscape).docx page 8 MO's of Benzene Benzene's extra stability cannot be explained by resonance alone, and so we must turn to Molecular Orbital theory for a fuller answer. Benzene has 6 planar sp2 carbons, and therefore each carbon has an unhybridized p orbital. These p orbitals are perfectly aligned for overlap (i.e. bonding, just like for a bond). These p orbitals create a continuous ring of orbitals above and below the plane of the carbon atoms. The 6 overlapping p orbitals create a cyclic system of molecular orbitals (i.e. a three dimensional system). Even though we have only seen two dimensional MO's previously (ethene, allyl systems, the same basic rules apply. 1) Six p orbitals are used in the benzene system, therefore six MO's are created. 2) The lowest energy MO is entirely bonding (constructive overlap between all adjacent p orbitals; no nodes). 3) The number of nodes increases as the MO's increase in energy. 4) The MO's must be divided between bonding and antibonding, with the possibility on non-bonding MO's in some cases. The 6 MO's for benzene can be drawn either in 2D or 3D projections
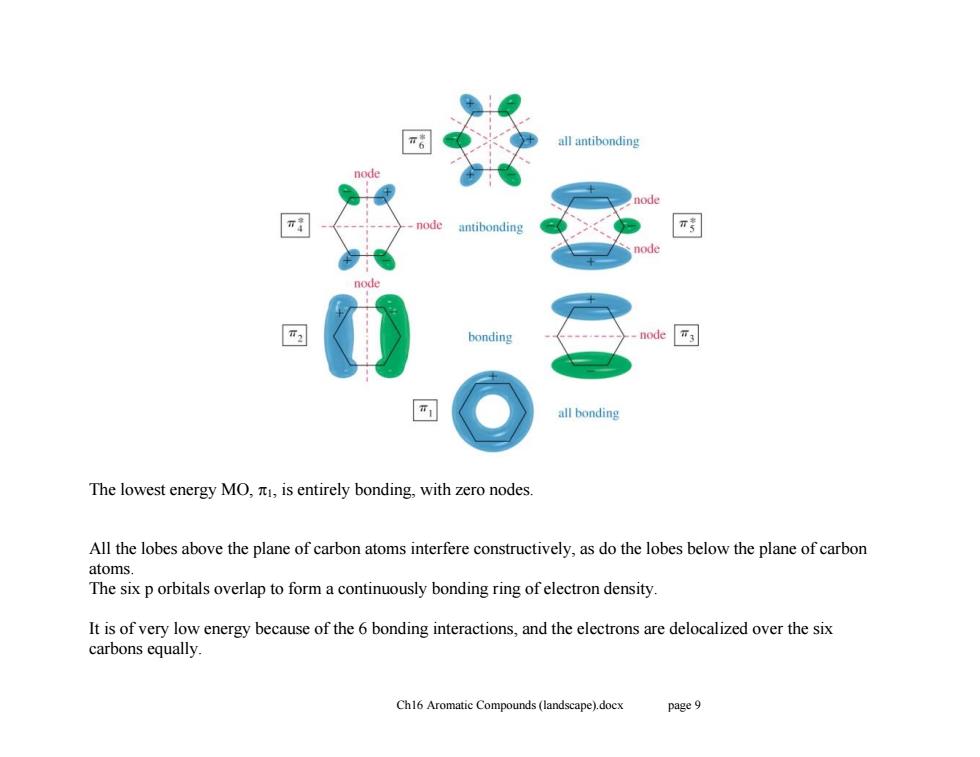
all antibonding antibonding π ode bonding node T3 all bonding The lowest energy MO,is entirely bonding,with zero nodes. All the lobes above the plane of carbon atoms interfere constructively,as do the lobes below the plane of carbon atoms. The six p orbitals overlap to form a continuously bonding ring of electron density. It is of very low energy because of the 6 bonding interactions,and the electrons are delocalized over the six carbons equally. Ch16 Aromatic Compounds(landscape).docx page 9
Ch16 Aromatic Compounds (landscape).docx page 9 The lowest energy MO, 1, is entirely bonding, with zero nodes. All the lobes above the plane of carbon atoms interfere constructively, as do the lobes below the plane of carbon atoms. The six p orbitals overlap to form a continuously bonding ring of electron density. It is of very low energy because of the 6 bonding interactions, and the electrons are delocalized over the six carbons equally
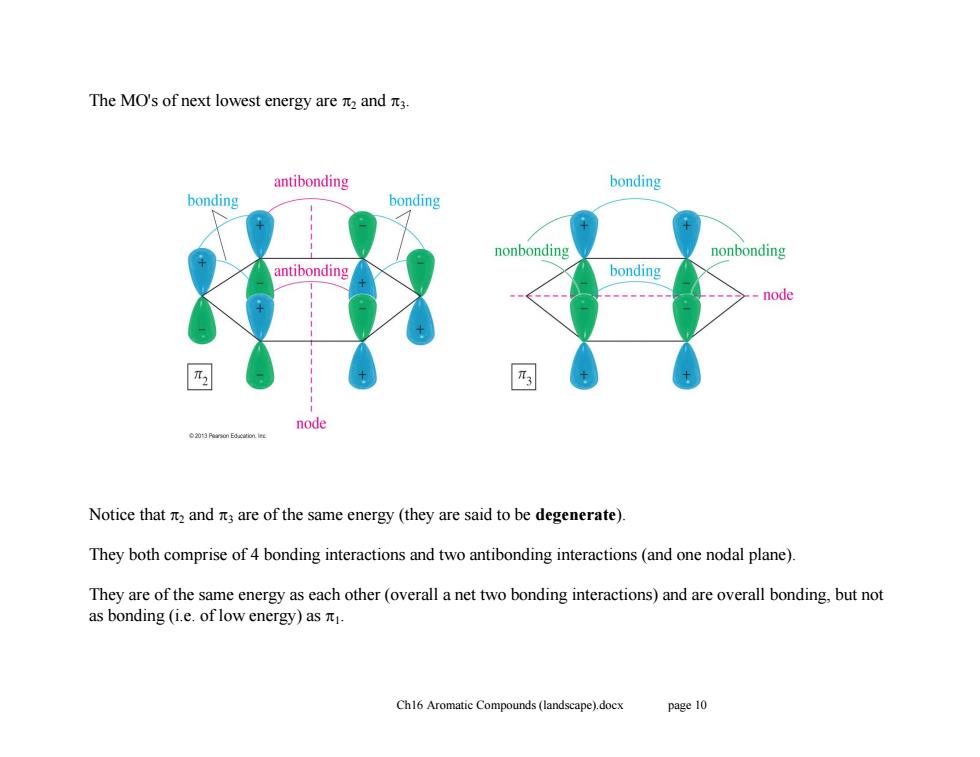
The MO's of next lowest energy are n and it3. antibonding bonding bonding bonding nonbonding nonbonding antibonding bonding --node node Notice that and are of the same energy(they are said to be degenerate). They both comprise of 4 bonding interactions and two antibonding interactions(and one nodal plane). They are of the same energy as each other(overall a net two bonding interactions)and are overall bonding,but not as bonding(i.e.of low energy)as元, Ch16 Aromatic Compounds(landscape).docx page 10
Ch16 Aromatic Compounds (landscape).docx page 10 The MO's of next lowest energy are 2 and 3. Notice that 2 and 3 are of the same energy (they are said to be degenerate). They both comprise of 4 bonding interactions and two antibonding interactions (and one nodal plane). They are of the same energy as each other (overall a net two bonding interactions) and are overall bonding, but not as bonding (i.e. of low energy) as 1