
窗 现代有机合成Ⅱ(B2) 中基本概念Basic concep 姚祝军 合成类型及其特点 南京大学化学化工学院 .线性合成(Linear Synthesis)) Camptothecin synthesis(1) made thoughres 0头 2.汇聚式合成(Convergent Synthesis) Camptothecin synthesis(2) ovorall yiol 0 6云g=了学饮司 antages cps色total steps) 1
1 姚祝军 南京大学化学化工学院 Email: yaoz@nju.edu.cn 2016年4月 1 现代有机合成 II (B2) 南京大学化学化工学院 2015级研究生课程 基本概念 Basic concepts 2 合成类型及其特点 © 2016 YZJ@NJU 1. 线性合成(Linear Synthesis) 3 The target compound is made through a series of linear transformations. A B 5 steps Overall yield 90%/step 59% 70%/step 17% © 2016 YZJ@NJU Camptothecin synthesis (1) Zhou, H.-B.; et al. Org. Lett. 2007, 9, 2003-2006; Liu, G.-S.; et al. Org. Lett. 2008, 10, 5393-6. 4 13-14 steps © 2016 YZJ@NJU 2. 汇聚式合成(Convergent Synthesis) 5 Advantages 1. Shorter longest linear steps (in same total steps); 2. Higher overall yields; 3. Simpler to execute; 4. Better material balance and supply. Individually prepared compounds are convergently brought together to make the target compound. C D E F B 5 steps overall yield 90%/step 73% 70%/step 34% © 2016 YZJ@NJU Camptothecin synthesis (2) 6 Xu, P.; Chen, D.-S.; Xi, J.; Yao, Z.-J. Chem. Asian J. 2015, 10, 976-981. 8-9 longest linear steps
上汇聚式合成(Convergent Synthesis) 集2发散性合成(Div5 Syathes司 Triply Convergent Synthesis c E F -I G H Family synthesis 通h 发散性合成(Divergent Synthesis) 集立体发散性合成 地地地地 二地地地 地批一 战 燃 Collective synthesis Q1.MacMillan's catalytic cycles 一石多鸟 2
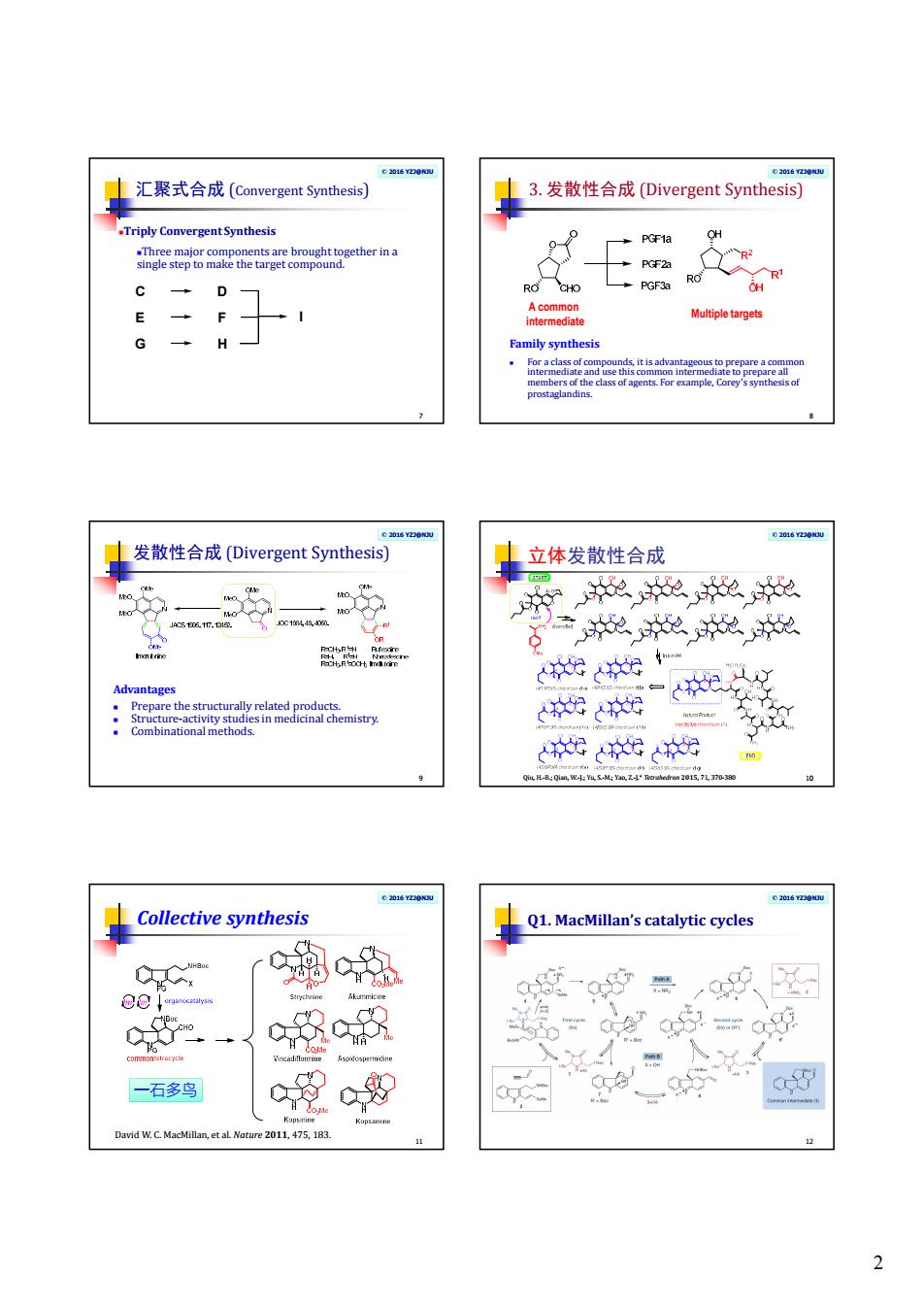
2 © 2016 YZJ@NJU 汇聚式合成 (Convergent Synthesis) 7 Triply Convergent Synthesis Three major components are brought together in a single step to make the target compound. C D E F G H I © 2016 YZJ@NJU 3. 发散性合成 (Divergent Synthesis) 8 Family synthesis For a class of compounds, it is advantageous to prepare a common intermediate and use this common intermediate to prepare all members of the class of agents. For example, Corey’s synthesis of prostaglandins. A common intermediate Multiple targets © 2016 YZJ@NJU 发散性合成 (Divergent Synthesis) 9 Advantages Prepare the structurally related products. Structure-activity studies in medicinal chemistry. Combinational methods. © 2016 YZJ@NJU 立体发散性合成 Qiu, H.-B.; Qian, W.-J.; Yu, S.-M.; Yao, Z.-J.* Tetrahedron 2015, 71, 370-380 10 © 2016 YZJ@NJU Collective synthesis 11 David W. C. MacMillan, et al. Nature 2011, 475, 183. 一石多鸟 © 2016 YZJ@NJU Q1. MacMillan’s catalytic cycles 12
4.全合成(Total Synthesis) 美半合成e9nc间 dpend2nwyoaethatohetargetnoe 8 Hr t al Angew Ch20042,46 Pharmaceutical production Semi-synthesis new drugs 。Taxolf的半合成(Holton@Florida State U)】 6.形式合成(Formal Synthesis) 全合成s形式合成 ons were also 3
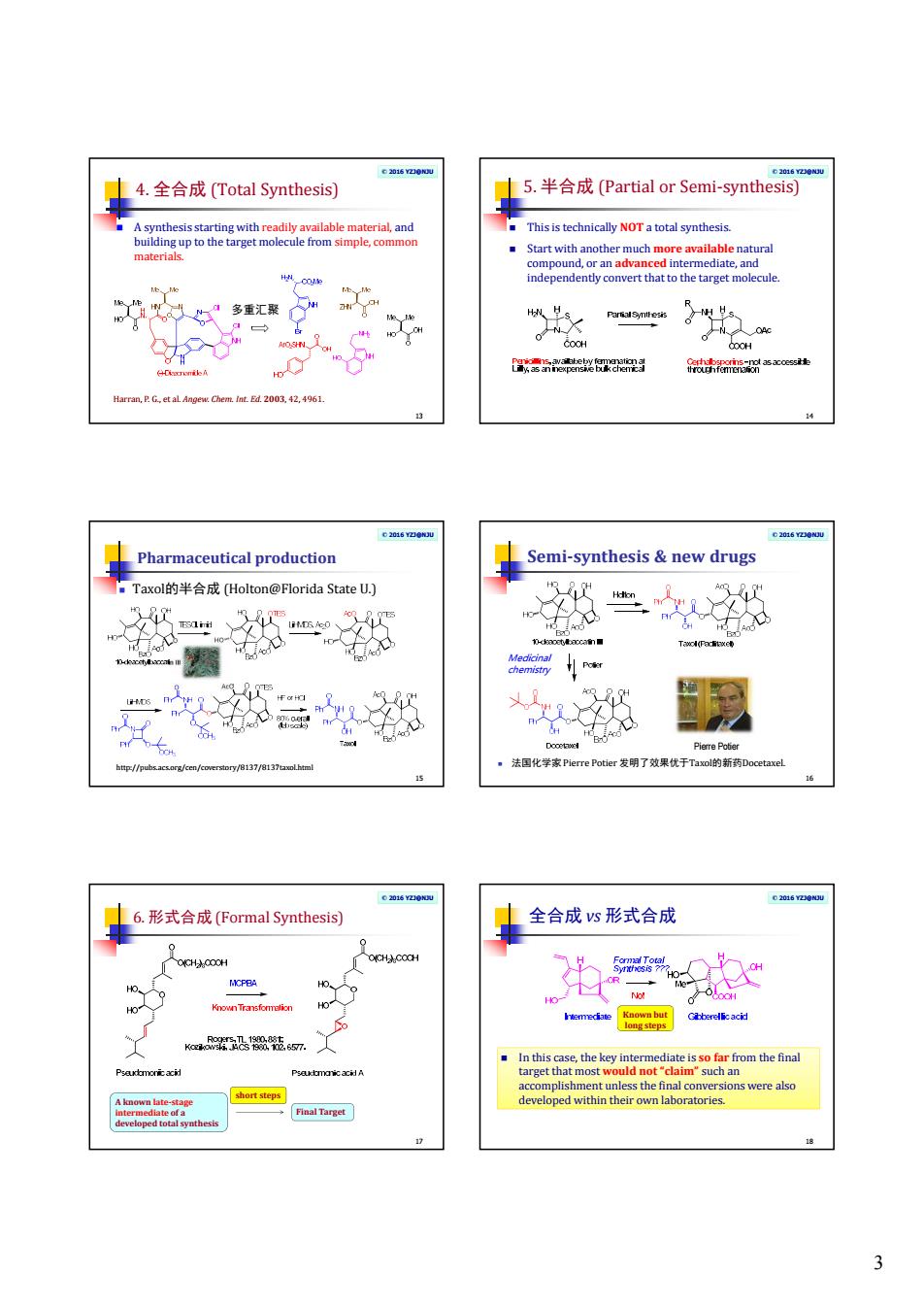
3 © 2016 YZJ@NJU 4. 全合成 (Total Synthesis) 13 A synthesis starting with readily available material, and building up to the target molecule from simple, common materials. Harran, P. G., et al. Angew. Chem. Int. Ed. 2003, 42, 4961. 多重汇聚 © 2016 YZJ@NJU 5. 半合成 (Partial or Semi-synthesis) 14 This is technically NOT a total synthesis. Start with another much more available natural compound, or an advanced intermediate, and independently convert that to the target molecule. © 2016 YZJ@NJU Pharmaceutical production Taxol的半合成 (Holton@Florida State U.) 15 http://pubs.acs.org/cen/coverstory/8137/8137taxol.html © 2016 YZJ@NJU Semi-synthesis & new drugs 16 法国化学家 Pierre Potier 发明了效果优于Taxol的新药Docetaxel. Pierre Potier Medicinal chemistry © 2016 YZJ@NJU 6. 形式合成 (Formal Synthesis) 17 A known late-stage intermediate of a developed total synthesis Final Target short steps © 2016 YZJ@NJU 全合成 vs 形式合成 18 In this case, the key intermediate is so far from the final target that most would not “claim” such an accomplishment unless the final conversions were also developed within their own laboratories. Known but long steps

上7.生源模拟合成 Synthesis of Tropinone The reaction must be capable ofoccurring. Steroid synthesis Proposed yciation of 2学名。 Squalene cyclases Ideal Reactions 长e长628a,8s
4 © 2016 YZJ@NJU 7. 生源模拟合成 19 Biomimetic total synthesis is believed that one can effectively mimic the conditions provided by nature, and conduct the same reaction in a flask. Two important considerations The reaction must be capable of occurring. The biogenetic process is under a great deal of control (enzymatic) and a similar level of control in lab may be difficult, but necessary. © 2016 YZJ@NJU Synthesis of Tropinone 20 Robinson, R. J. Chem. Soc. 1917, 111, 762-769. Willstater, R. Liebigs. Ann. Chem. 1903, 326, 1-42. Robert Robinson • His synthesis of tropinone, a precursor of cocaine, in 1917 was not only a big step in alkaloid chemistry, but also showed that tandem reactions in a one-pot synthesis are capable of forming bicyclic molecules. http://en.wikipedia.org/wiki/Robert_Robinson_(organic_chemist) • Nobel laureate recognised in 1947 for his research on plant dyestuffs (anthocyanins) and alkaloids. The First Biomimetic Synthesis © 2016 YZJ@NJU Steroid synthesis 21 © 2016 YZJ@NJU Proposed cyclization of squalene 22 Langdon, R. G.; Bloch, K. J. Am. Chem. Soc. 1952, 74, 1969-1870. Woodward, R. B.; Bloch, K. J. Am. Chem. Soc. 1953, 75, 2023-2024. © 2016 YZJ@NJU Squalene cyclases 23 Wendt, K. U.; Poralla, K.; Schulz, G. E. Science 1997, 277, 1811-1815. Hoshino, T.; Sato, T. Chem. Commun. 2002, 4, 291-301. © 2016 YZJ@NJU Ideal Reactions 24 W. S. Johnson C. Heathcock “The basic assumption to this approach is that Nature is the quintessential process chemist. We think that the molecular frameworks of most natural products arise by intrinsically favorable chemical pathways-favorable enough that the skeleton could have arisen by a nonenzymatic reaction in the primitive organism” - Clayton Heathcock • Minimum enzymatic assistance • Architectural self construction
Total synthesis of azonazine Synthesis of manzamines 解兰6 除「奇 199154-361 Experimental Nternative hypotheses a5等 1 A semi-biomimic approach 反合成分析 Retrosynthetic Analysis lack E Baldwin etal.Chem far/1999.5.3154-3161 5
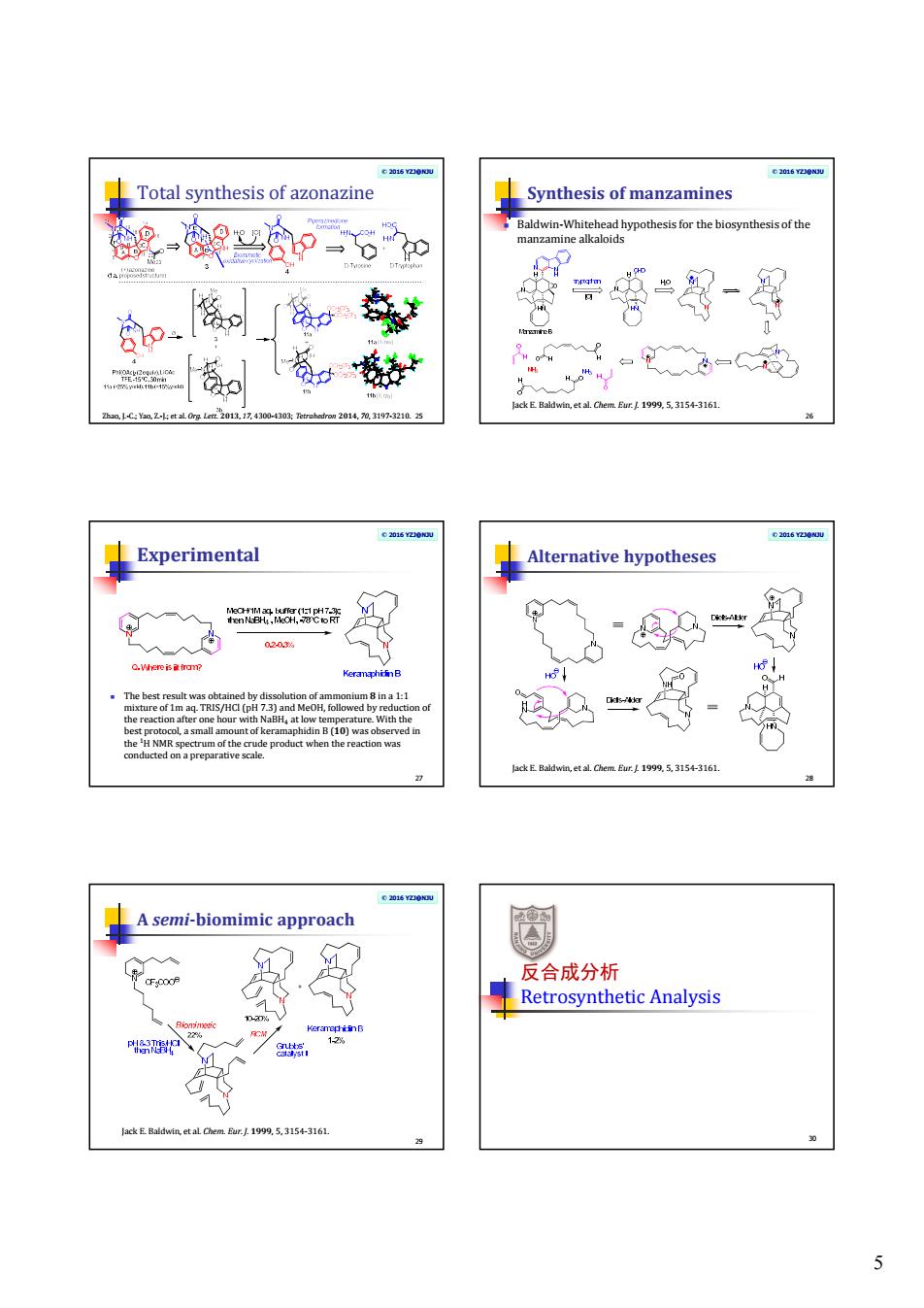
5 © 2016 YZJ@NJU Total synthesis of azonazine Zhao, J.-C.; Yao, Z.-J.; et al. Org. Lett. 2013, 17, 4300-4303; Tetrahedron 2014, 70, 3197-3210. 25 © 2016 YZJ@NJU Synthesis of manzamines Baldwin-Whitehead hypothesis for the biosynthesis of the manzamine alkaloids 26 Jack E. Baldwin, et al. Chem. Eur. J. 1999, 5, 3154-3161. © 2016 YZJ@NJU Experimental The best result was obtained by dissolution of ammonium 8 in a 1:1 mixture of 1m aq. TRIS/HCl (pH 7.3) and MeOH, followed by reduction of the reaction after one hour with NaBH4 at low temperature. With the best protocol, a small amount of keramaphidin B (10) was observed in the 1H NMR spectrum of the crude product when the reaction was conducted on a preparative scale. 27 © 2016 YZJ@NJU Alternative hypotheses 28 Jack E. Baldwin, et al. Chem. Eur. J. 1999, 5, 3154-3161. © 2016 YZJ@NJU A semi-biomimic approach 29 Jack E. Baldwin, et al. Chem. Eur. J. 1999, 5, 3154-3161. 反合成分析 Retrosynthetic Analysis 30