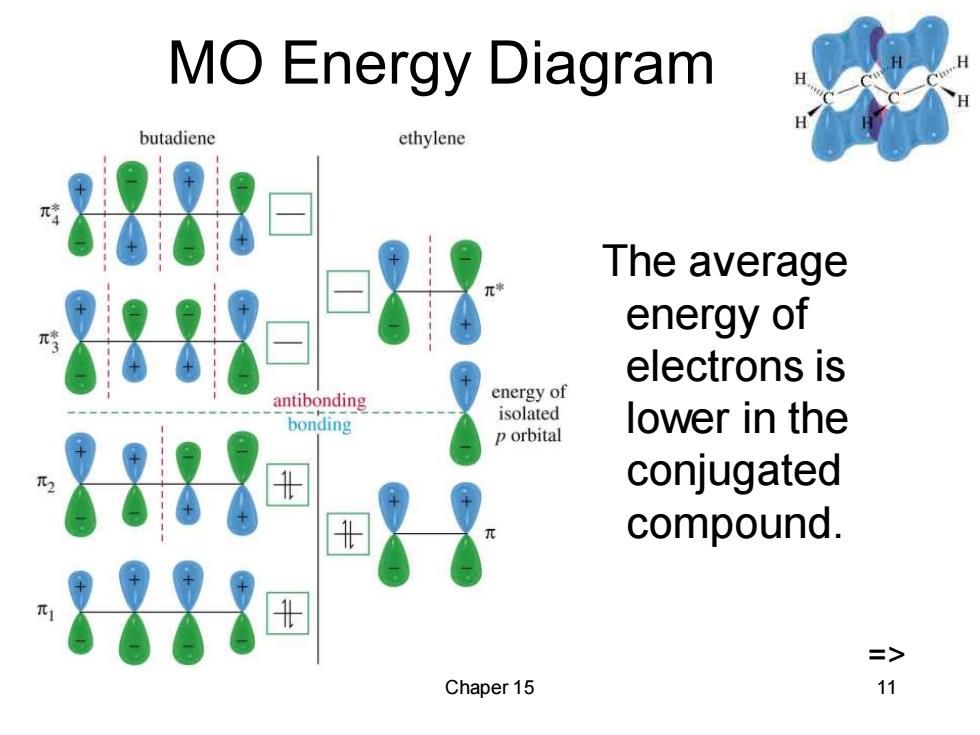
MO Energy Diagram butadiene ethylene The average energy of electrons is antibonding energy of isolated bonding lower in the p orbital 88 conjugated compound. => Chaper 15 11
Chaper 15 11 MO Energy Diagram The average energy of electrons is lower in the conjugated compound. =>
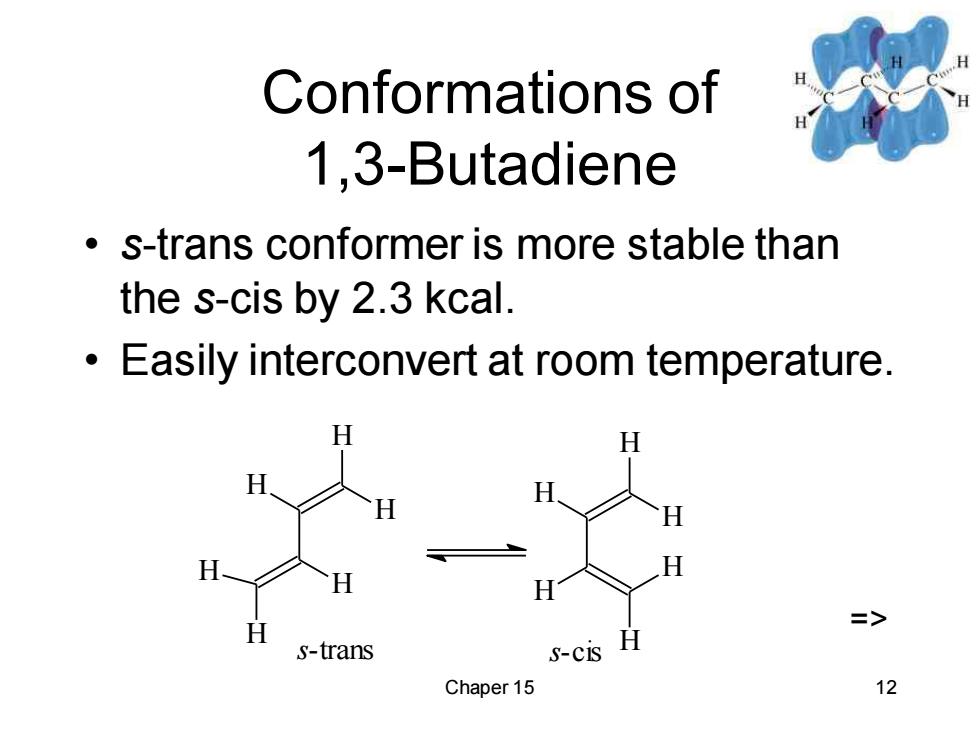
Conformations of 1,3-Butadiene s-trans conformer is more stable than the s-cis by 2.3 kcal. Easily interconvert at room temperature. s-trans S-CIS Chaper 15 12
Chaper 15 12 Conformations of 1,3-Butadiene • s-trans conformer is more stable than the s-cis by 2.3 kcal. • Easily interconvert at room temperature. H H H H H H s-trans s-cis H H H H H H =>
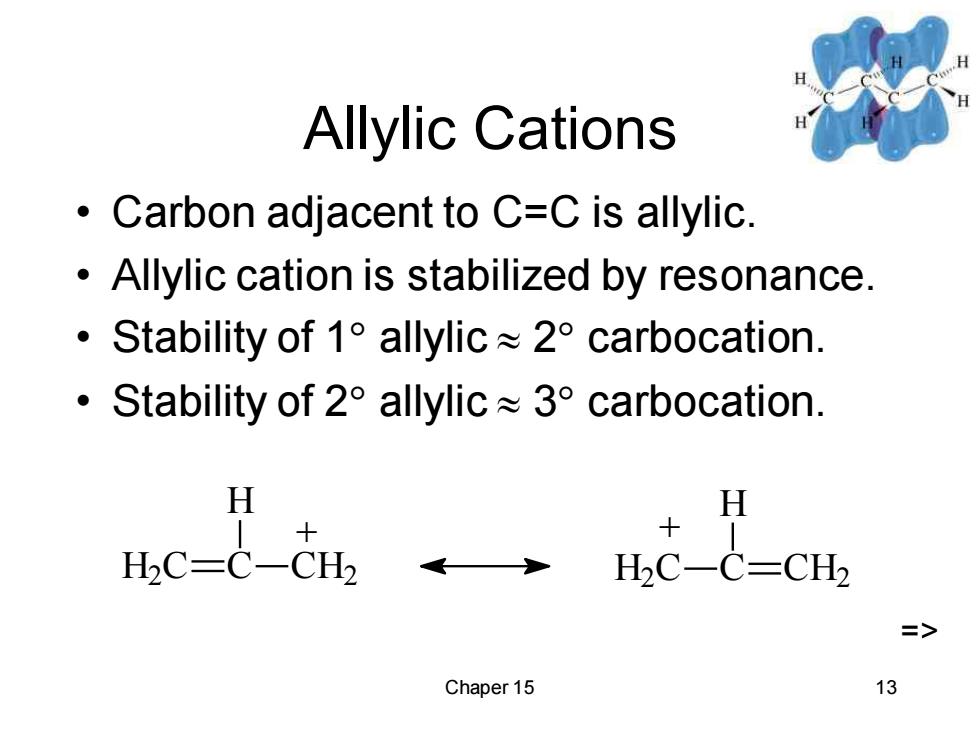
Allylic Cations Carbon adjacent to C=C is allylic. Allylic cation is stabilized by resonance. 。 Stability of1 allylic≈2°carbocation. ·Stability of2°allylic≈3°carbocation. H H H2C=C-CH2 H2C-C=CH2 => Chaper 15 13
Chaper 15 13 Allylic Cations • Carbon adjacent to C=C is allylic. • Allylic cation is stabilized by resonance. • Stability of 1 allylic 2 carbocation. • Stability of 2 allylic 3 carbocation. H2 C C H CH2 + H2 C C H CH2 + =>