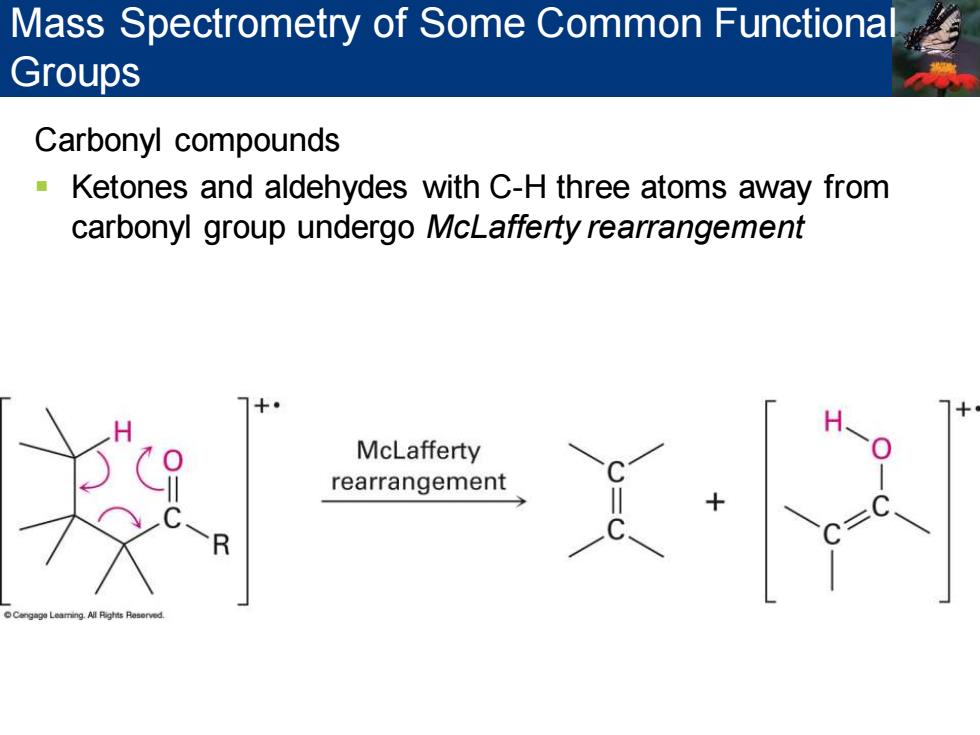
Mass Spectrometry of Some Common Functiona Groups Carbonyl compounds Ketones and aldehydes with C-H three atoms away from carbonyl group undergo McLafferty rearrangement McLafferty rearrangement
Carbonyl compounds ▪ Ketones and aldehydes with C-H three atoms away from carbonyl group undergo McLafferty rearrangement Mass Spectrometry of Some Common Functional Groups
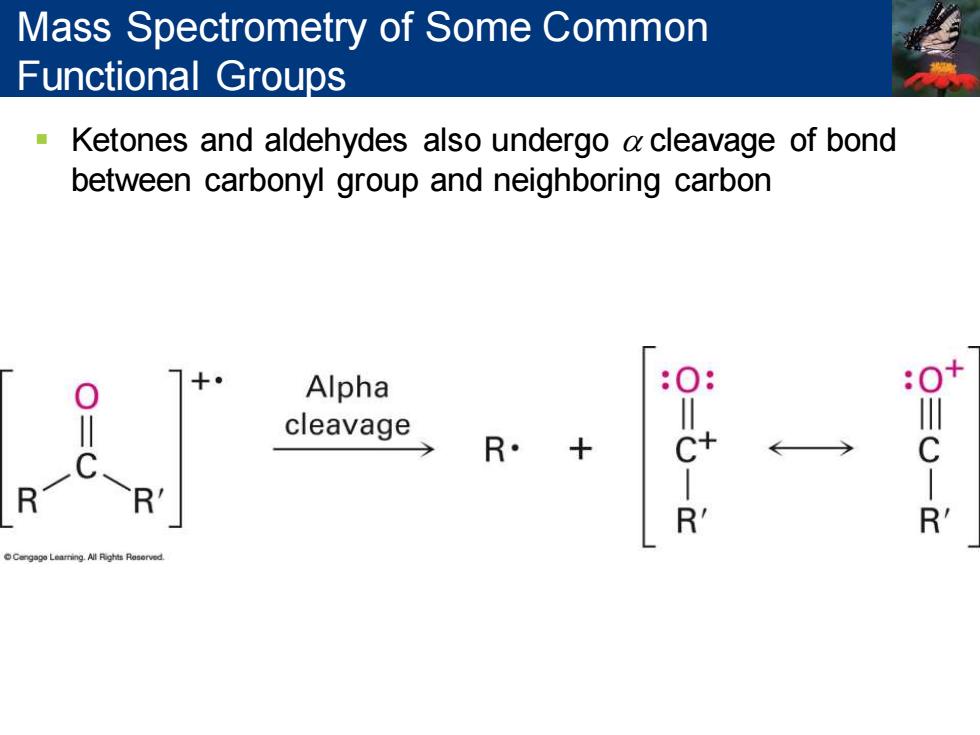
Mass Spectrometry of Some Common Functional Groups Ketones and aldehydes also undergo a cleavage of bond between carbonyl group and neighboring carbon Alpha cleavage R
▪ Ketones and aldehydes also undergo a cleavage of bond between carbonyl group and neighboring carbon Mass Spectrometry of Some Common Functional Groups
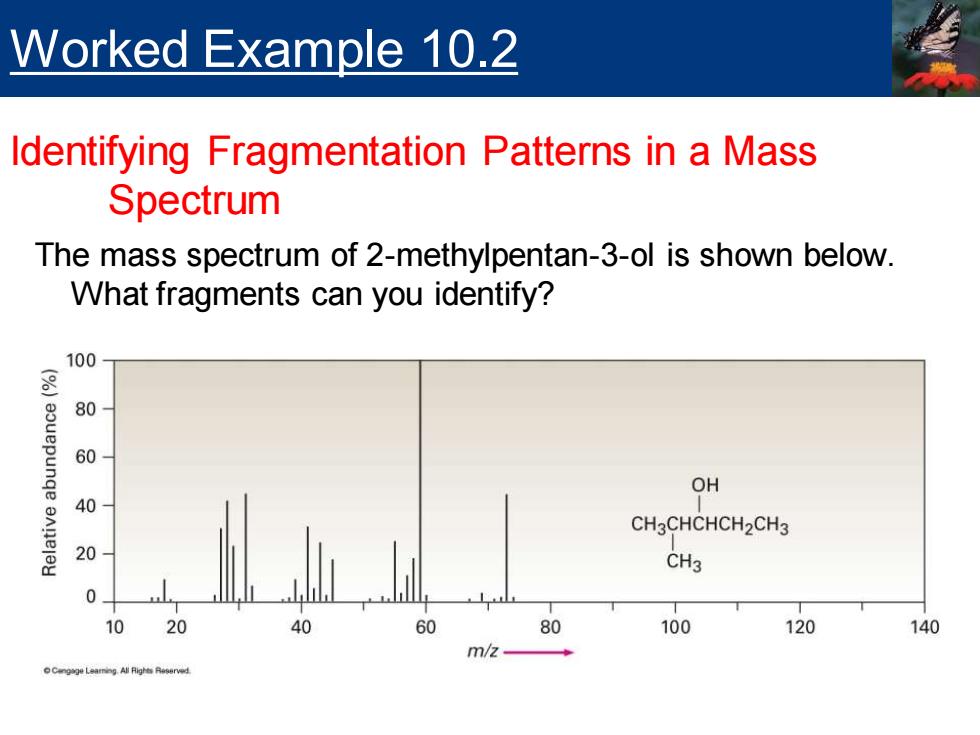
Worked Example 10.2 Identifying Fragmentation Patterns in a Mass Spectrum The mass spectrum of 2-methylpentan-3-ol is shown below. What fragments can you identify? 100 8 80 60 OH 40 CH3CHCHCH2CH3 20 CH3 0 10 20 60 80 100 120 140 m/2
The mass spectrum of 2-methylpentan-3-ol is shown below. What fragments can you identify? Worked Example 10.2 Identifying Fragmentation Patterns in a Mass Spectrum

Worked Example 10.2 Identifying Fragmentation Patterns in a Mass Spectrum Strategy Calculate the mass of the molecular ion,and identify the functional groups in the molecule. Then write the fragmentation processes you might expect,and compare the masses of the resultant fragments with the peaks present in the spectrum
Strategy ▪ Calculate the mass of the molecular ion, and identify the functional groups in the molecule. Then write the fragmentation processes you might expect, and compare the masses of the resultant fragments with the peaks present in the spectrum. Worked Example 10.2 Identifying Fragmentation Patterns in a Mass Spectrum
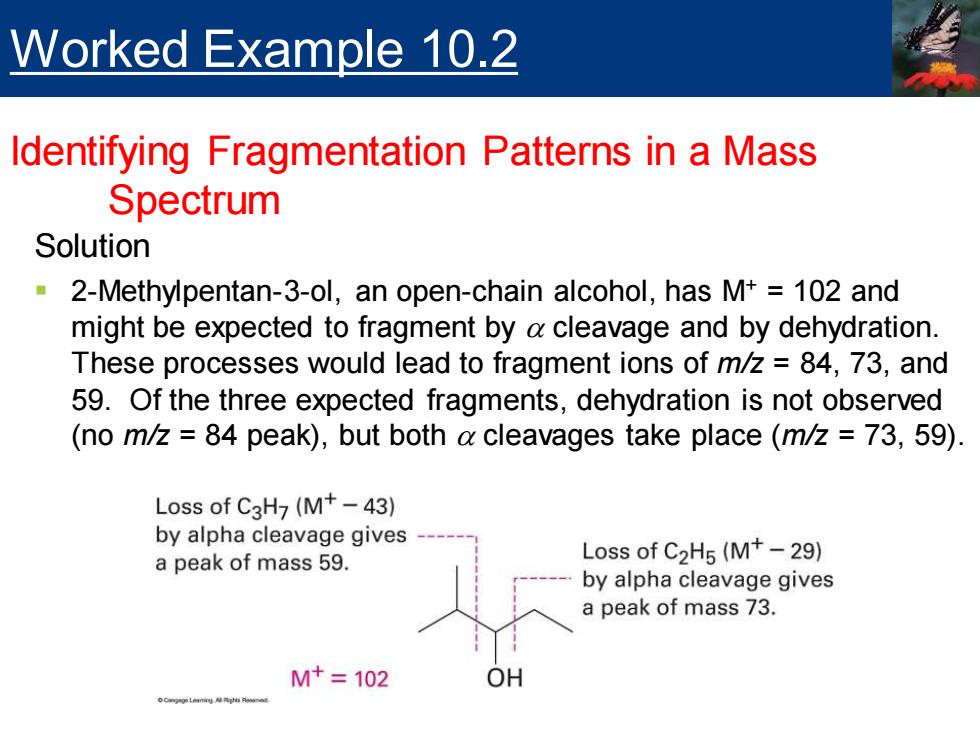
Worked Example 10.2 Identifying Fragmentation Patterns in a Mass Spectrum Solution 2-Methylpentan-3-ol,an open-chain alcohol,has M+102 and might be expected to fragment by a cleavage and by dehydration. These processes would lead to fragment ions of m/z=84,73,and 59.Of the three expected fragments,dehydration is not observed (no m/z=84 peak),but both a cleavages take place(m/z 73,59) Loss of C3H7(M+-43) by alpha cleavage gives a peak of mass 59. Loss of C2H5(M+-29) by alpha cleavage gives a peak of mass 73. M+=102 OH
Solution ▪ 2-Methylpentan-3-ol, an open-chain alcohol, has M+ = 102 and might be expected to fragment by a cleavage and by dehydration. These processes would lead to fragment ions of m/z = 84, 73, and 59. Of the three expected fragments, dehydration is not observed (no m/z = 84 peak), but both a cleavages take place (m/z = 73, 59). Worked Example 10.2 Identifying Fragmentation Patterns in a Mass Spectrum