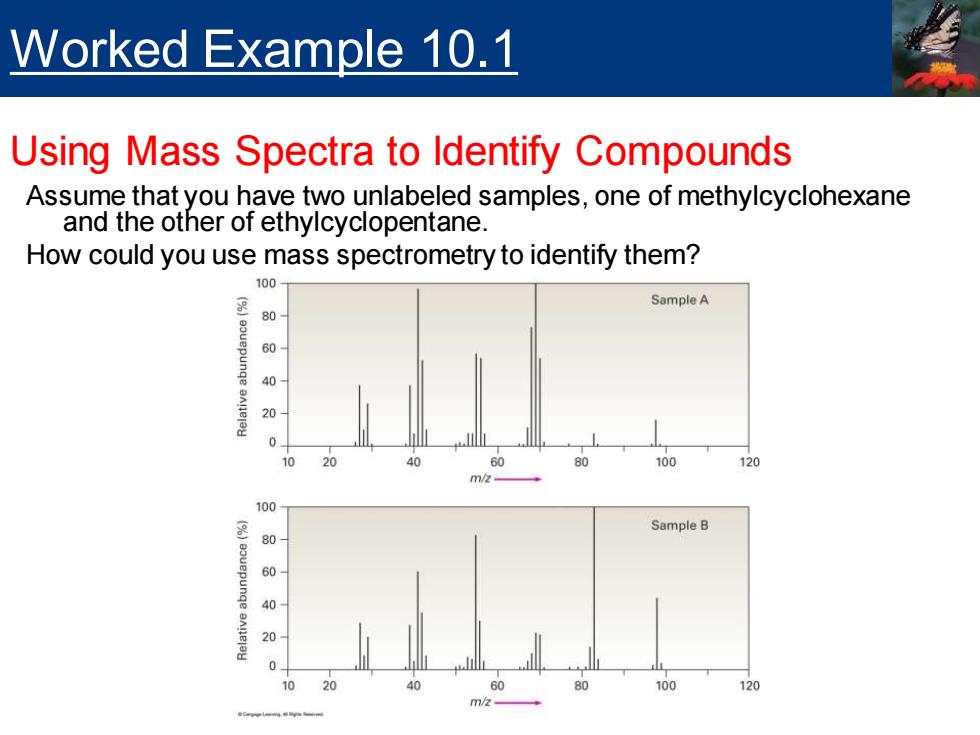
Worked Example 10.1 Using Mass Spectra to Identify Compounds Assume that you have two unlabeled samples,one of methylcyclohexane and the other of ethylcyclopentane. How could you use mass spectrometry to identify them? 100 SampleA 80 40 20 100 120 m/ Sample B 80 20
Assume that you have two unlabeled samples, one of methylcyclohexane and the other of ethylcyclopentane. How could you use mass spectrometry to identify them? Worked Example 10.1 Using Mass Spectra to Identify Compounds
Worked Example 10.1 Using Mass Spectra to Identify Compounds Strategy Look at the two possible structures and determine how they differ.Then think about how any of these differences in structure might give rise to differences in mass spectra.Methylcyclohexane, for instance,has a-CH3 group,and ethylcyclopentane has a -CH2CH3 group,which should affect the fragmentation process
Strategy ▪ Look at the two possible structures and determine how they differ. Then think about how any of these differences in structure might give rise to differences in mass spectra. Methylcyclohexane, for instance, has a –CH3 group, and ethylcyclopentane has a –CH2CH3 group, which should affect the fragmentation process. Worked Example 10.1 Using Mass Spectra to Identify Compounds
Worked Example 10.1 Using Mass Spectra to ldentify Compounds Solution The mass spectra of both samples show molecular ions at M+=98,corresponding to C-H14,but the two spectra differ in their fragmentation patterns.Sample A has its base peak at m/z=69,corresponding to the loss of a CH2CH3 group(29 mass units),but B has a rather small peak at mz =69.Sample B shows a base peak at mz 83,corresponding to the loss of a CHa group(15 mass units),but sample A has only a small peak at m/z 83.We can therefore be reasonably certain that A is ethylcyclopentane and B is methylcyclohexane
Solution ▪ The mass spectra of both samples show molecular ions at M+ = 98, corresponding to C7H14, but the two spectra differ in their fragmentation patterns. Sample A has its base peak at m/z = 69, corresponding to the loss of a CH2CH3 group (29 mass units), but B has a rather small peak at m/z = 69. Sample B shows a base peak at m/z = 83, corresponding to the loss of a CH3 group (15 mass units), but sample A has only a small peak at m/z = 83. We can therefore be reasonably certain that A is ethylcyclopentane and B is methylcyclohexane. Worked Example 10.1 Using Mass Spectra to Identify Compounds
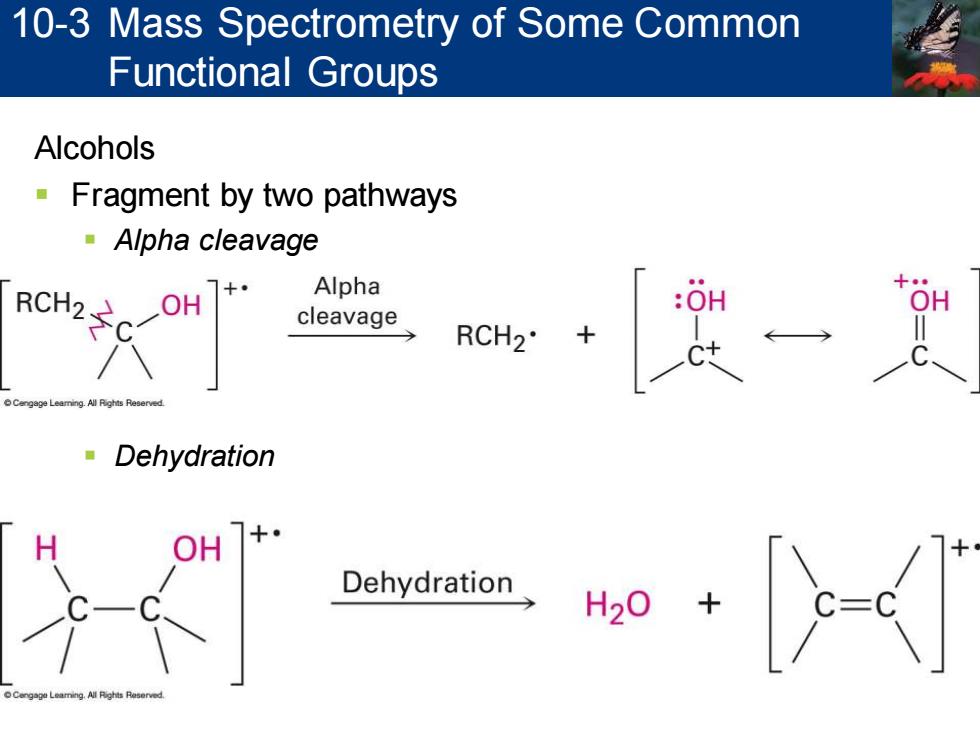
10-3 Mass Spectrometry of Some Common Functional Groups Alcohols Fragment by two pathways Alpha cleavage RCH2 Alpha cleavage RCH2 Dehydration Dehydration
Alcohols ▪ Fragment by two pathways ▪ Alpha cleavage ▪ Dehydration 10-3 Mass Spectrometry of Some Common Functional Groups
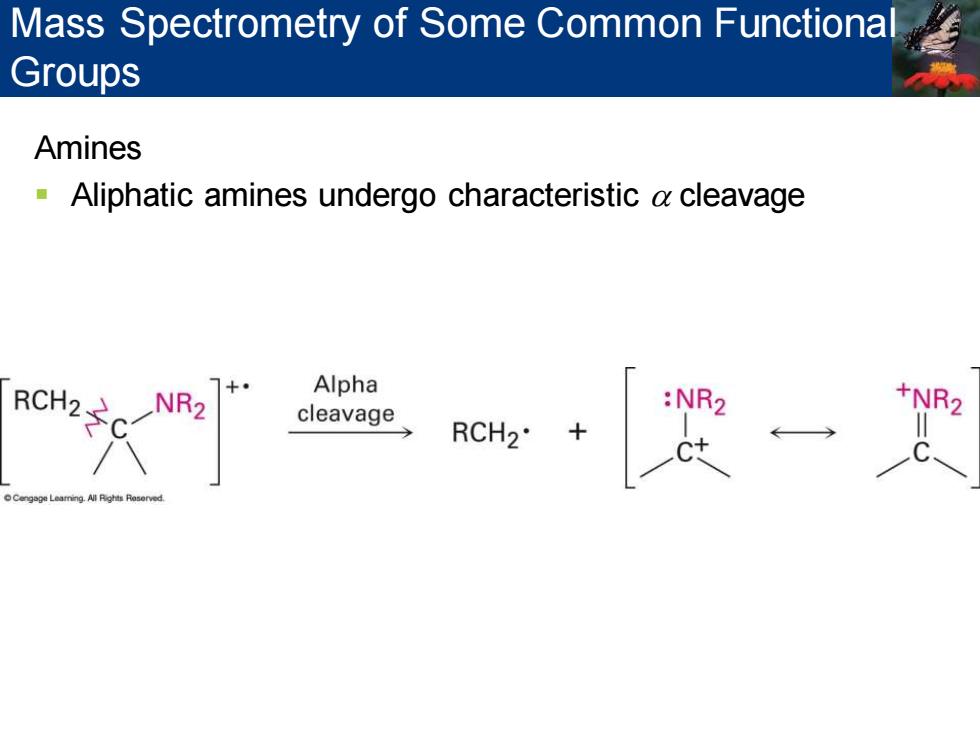
Mass Spectrometry of Some Common Functiona Groups Amines Aliphatic amines undergo characteristic a cleavage RCH2 NR Alpha ←
Amines ▪ Aliphatic amines undergo characteristic a cleavage Mass Spectrometry of Some Common Functional Groups