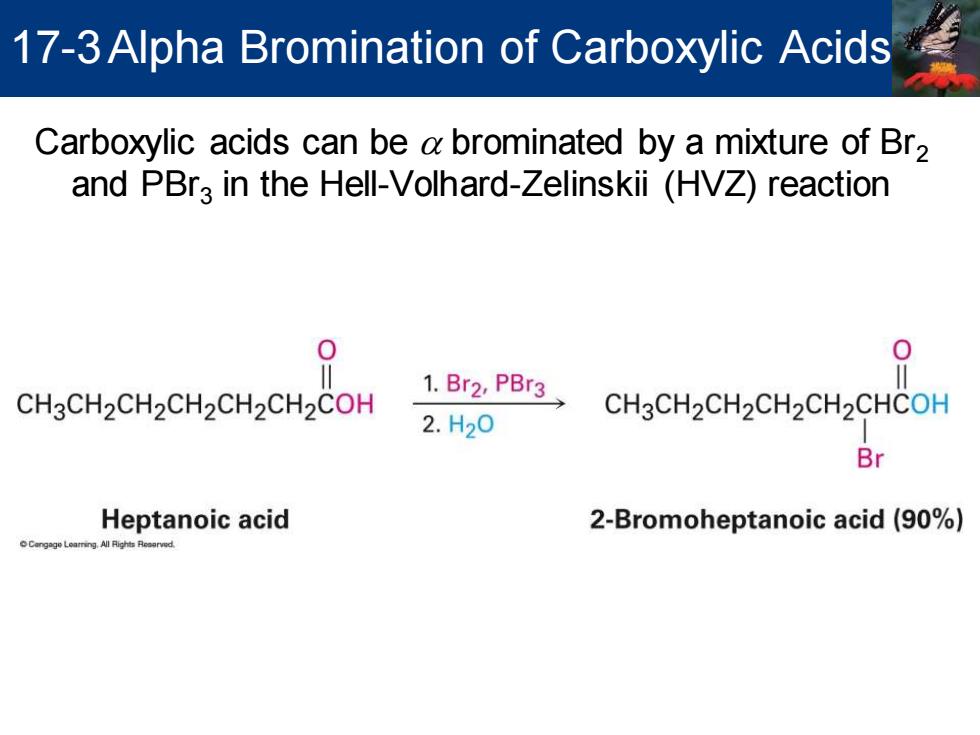
17-3 Alpha Bromination of Carboxylic Acids Carboxylic acids can be a brominated by a mixture of Br2 and PBra in the Hell-Volhard-Zelinskii (HVZ)reaction CH3CH2CH2CH2CH2CH2COH 1.Br2,PBr3 CH3CH2CH2CH2CH2CHCOH 2.H20 Br Heptanoic acid 2-Bromoheptanoic acid(90%)
Carboxylic acids can be a brominated by a mixture of Br2 and PBr3 in the Hell-Volhard-Zelinskii (HVZ) reaction 17-3Alpha Bromination of Carboxylic Acids

Alpha Bromination of Carboxylic Acids Mechanism involves a-substitution of an acid bromide enol HBr H PBr3 Br2 Carboxylic Acid bromide Acid bromide H20 acid enol B HBr OH H a-Bromo carboxylic acid Cangage Learring All Rights Reeorved
▪ Mechanism involves a-substitution of an acid bromide enol Alpha Bromination of Carboxylic Acids

17-4 Acidity of a Hydrogen Atoms:Enolate lon Formation Presence of neighboring carbonyl group increases the acidity of the ketone over the alkane by a factor H H 0f1040 Acetone Ethane (pKa=19.3) (pKa≈60) Proton abstraction from carbonyl occurs when the a C-H bond is oriented parallel to the p orbitals of the carbonyl group A carbon of the enolate ion has a p orbital that overlaps neighboring p orbitals of the carbonyl group Negative charge shared with oxygen atom by resonance Electron-rich sp-hybridized sp--hybridized Electron-rich
Presence of neighboring carbonyl group increases the acidity of the ketone over the alkane by a factor of 1040 Proton abstraction from carbonyl occurs when the a C-H bond is oriented parallel to the p orbitals of the carbonyl group A carbon of the enolate ion has a p orbital that overlaps neighboring p orbitals of the carbonyl group Negative charge shared with oxygen atom by resonance 17-4 Acidity of α Hydrogen Atoms: Enolate Ion Formation
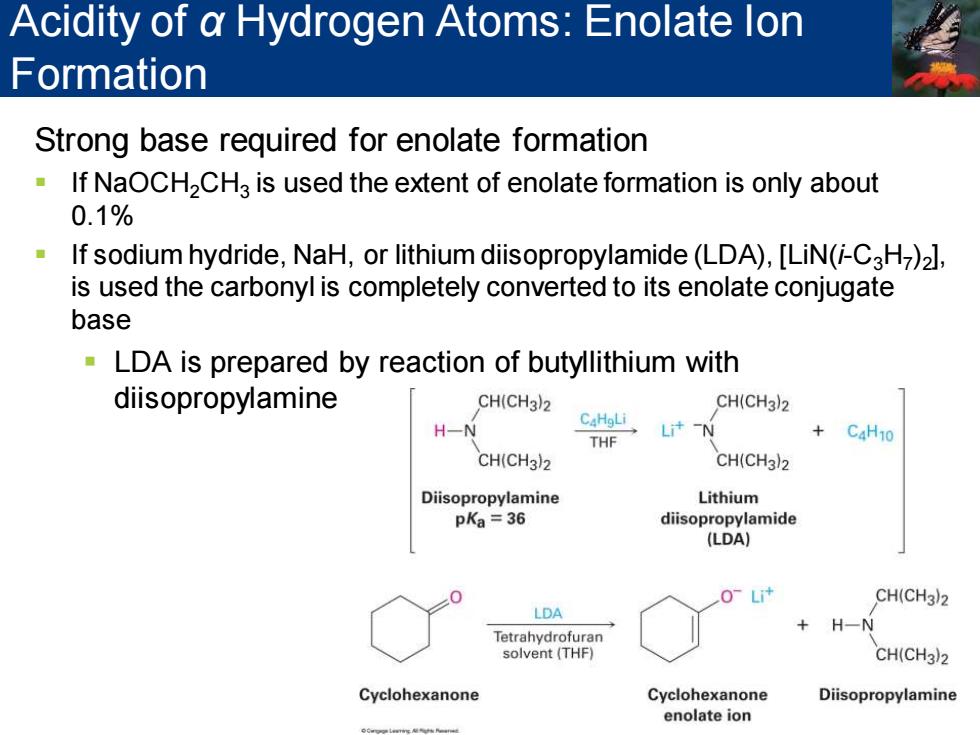
Acidity of a Hydrogen Atoms:Enolate lon Formation Strong base required for enolate formation If NaOCH2CHa is used the extent of enolate formation is only about 0.1% If sodium hydride,NaH,or lithium diisopropylamide(LDA),[LiN(-C3H)2], is used the carbonyl is completely converted to its enolate conjugate base LDA is prepared by reaction of butyllithium with diisopropylamine CH(CH3)2 CH(CH3)2 C4HgLi H一N Lit-N THF +C4H10 CH(CH3)2 CH(CH3)2 Diisopropylamine Lithium pKa =36 diisopropylamide (LDA) O-Li+ CH(CH3)2 LDA + H-N Tetrahydrofuran solvent(THF) CH(CH3)2 Cyclohexanone Cyclohexanone Diisopropylamine enolate ion
Strong base required for enolate formation ▪ If NaOCH2CH3 is used the extent of enolate formation is only about 0.1% ▪ If sodium hydride, NaH, or lithium diisopropylamide (LDA), [LiN(i-C3H7 )2 ], is used the carbonyl is completely converted to its enolate conjugate base ▪ LDA is prepared by reaction of butyllithium with diisopropylamine Acidity of α Hydrogen Atoms: Enolate Ion Formation

Acidity of a Hydrogen Atoms:Enolate lon Formation A C-H bond flanked by two carbonyl groups is even more acidic Enolate ion is stabilized by delocalization of negative charge over both carbonyl groups Pentane-2,4-dione has three resonance forms :0: H3C CH3 HH Pentane-2,4-dione (pKa 9) Base :O: H3C CH3 H3 -CH3 H3 CH
A C-H bond flanked by two carbonyl groups is even more acidic ▪ Enolate ion is stabilized by delocalization of negative charge over both carbonyl groups ▪ Pentane-2,4-dione has three resonance forms Acidity of α Hydrogen Atoms: Enolate Ion Formation